Annals of Circulation
Vascular Smooth Muscle Cells in the Branching of Renal Arteries
AN Gansburgsky1* and AV Yaltsev2
2Department of Pathological Anatomy with the Course of Clinical Pathology, Yaroslavl State Medical University, Russia
Cite this as
Gansburgsky AN, Yaltsev AV (2017) Vascular Smooth Muscle Cells in the Branching of Renal Arteries. Ann Circ 2(1): 008-012. DOI: 10.17352/ac.000005Histological, morphometric, cytophotometry and statistical methods studied isolated smooth muscle cells at sites of the renal arteries with different hemodynamic conditions in newborns. It is shown that the population of smooth muscle cells on the straight sections of the renal arteries represented subpopulations of small, medium and large mononuclear cells. In the area of the arteries blood flow dividers, characterized by turbulence, twists, recycling and complex distribution of shear stress on the vascular wall, there is a pronounced polymorphism in the structure of the arteries of the population. Smooth muscle cells in these hemodynamic conditions, undergo hypertrophy and hyperplasia polyploidization. Phenotypic modulation myocytes occurring in arteries with high branching, anastomosing, many turns may initiate the development of hypertension, heart failure, ischemia, diabetes and obesity.
Abbreviations
SM: Smooth Muscle Cells; DNA: Deoxyribo Nucleic Acid; MCM: Micrometer; NM: Nanometer
Introduction
Areas of vascular branching attracted the attention of researchers since the mid 50-ies of the last century [1, 2]. Dividers blood flow correspond to areas of increased permeability of the endothelial lining and are prone to the formation of atheroma [3, 4]. As the analysis of the literature [5], the least studied structural component of the vascular wall in hemodynamic stress zone are smooth myocytes (SM). It is known that SM can acquire branched form, and their contact with the basement membrane of endothelial cells alone [6]. Hemodynamic conditions may be a factor influencing the properties of the SM of the vascular wall [7].
The purpose of work
Morphometric and cytophotometry analysis isolated smooth muscle cells in arteries areas with different hemodynamic conditions.
Material and Methods
We studied the right and left renal arteries in the branch at the gates of the body 26 newborn infants who died of cerebrovascular accidents in case of damage of the skull bones. For comparison, the study area is the same vascular blood flow dividers. Specimens were fixed in 10% neutral formalin, 0.1 M phosphate buffer at pH 7.0. Isolated smooth muscle cells (SM) was prepared by alkaline cell dissociation and stained with hematoxylin and eosin Feulgen (hydrolysis of a 5 N HCl at 370 C 12 minutes). We counted the relative abundance of two-nuclear elements, screw ocular micrometer measured linear parameters of the nucleus and cytoplasm of single-core and dual-core SM, as well as calculate their area and volume. The level of DNA in the nuclei of single- and dual-core assayed for SM cytophotometry “MIF-K” (Moscow State University) at a wavelength of 580 nm. Quantitative data were processed by the method of variation statistics.
Results of the Study
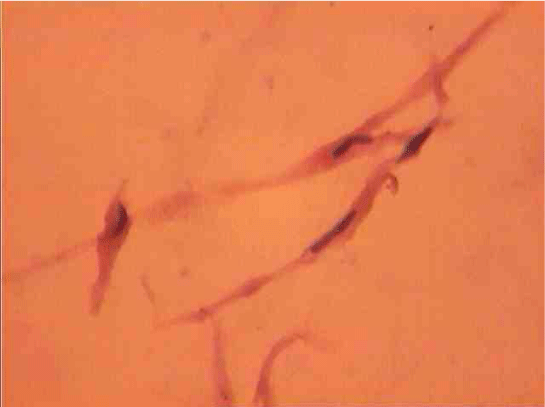
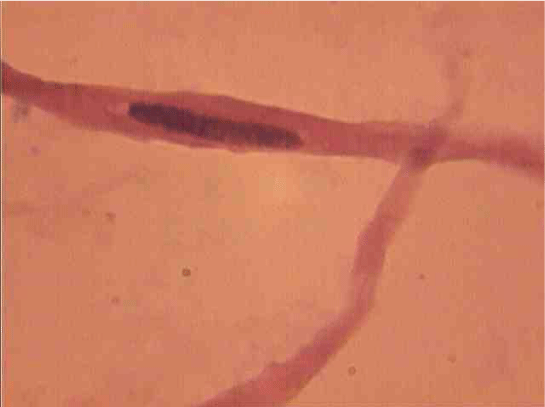
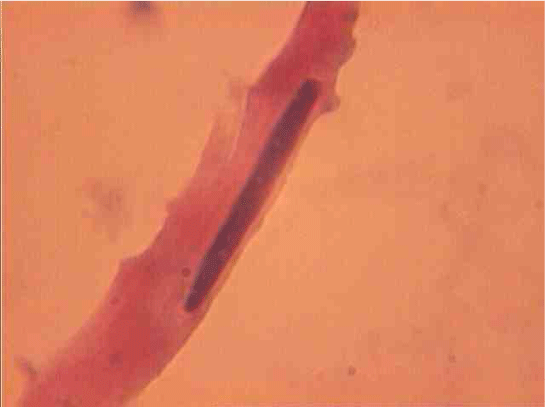
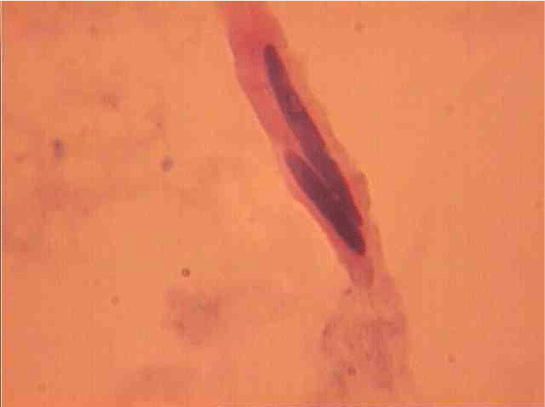
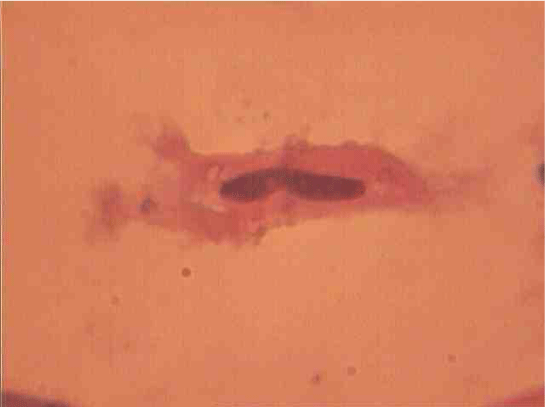
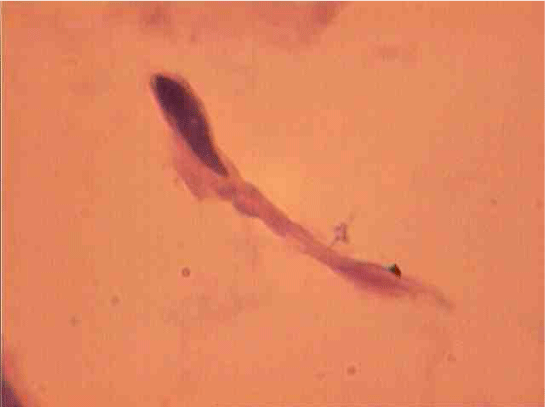
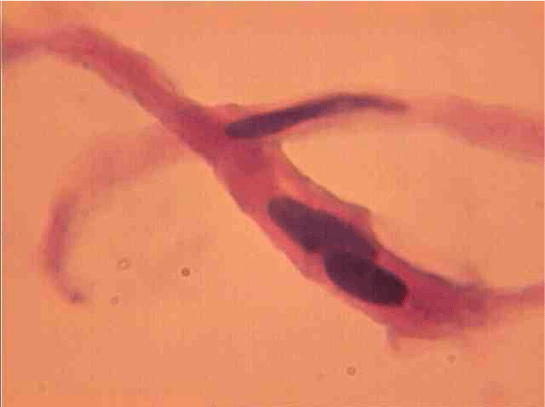
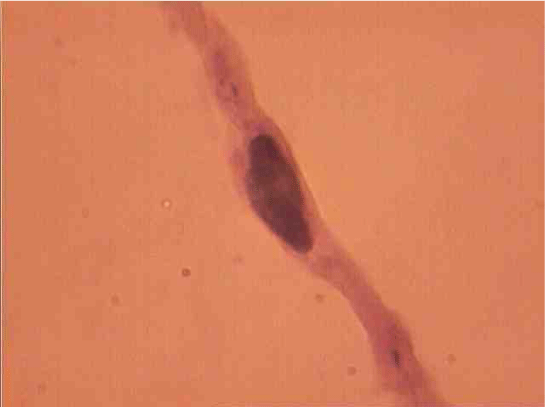
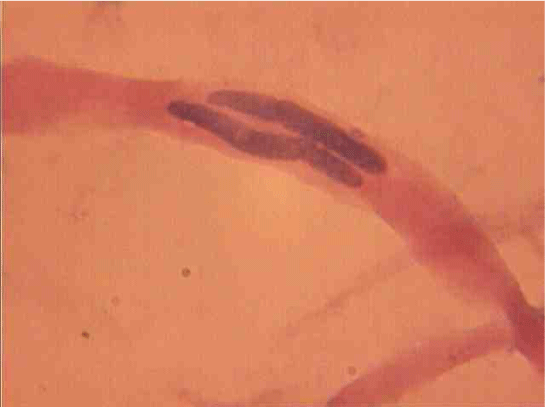
Population structure of smooth muscle cells of the renal arteries of newborn children on the straight sections of the vessel and presented heterogeneous, mostly single-core elements (Figure 1). The morphometric analysis helps identify a subpopulation of small (up to 1000 mcm3 to 24.8% - (Figure 2)), medium (1000 - 3000 mcm3 to 53.4% - (Figure 3)) and larger GM (more than 3000 mcm3 21.8 % - (Figure 4)). It was also found a small amount (0.2%) dual-core forms of SM (Figure 5). Cytophotometry analysis shows that SM population comprises mononuclear preferably diploid elements (Figure 6).
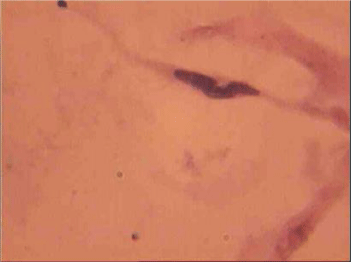
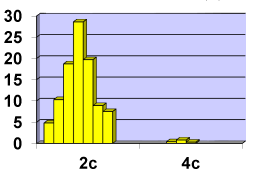
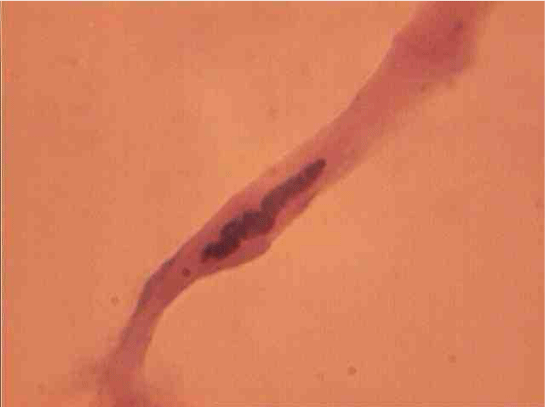
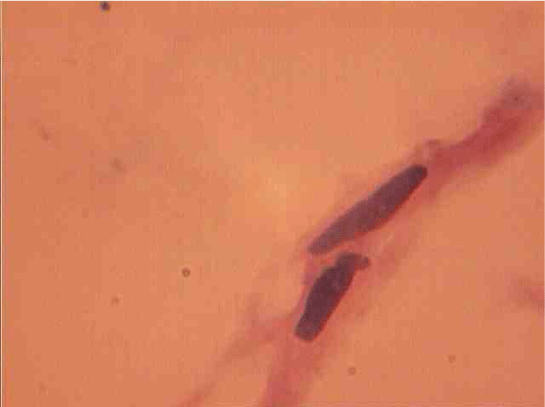
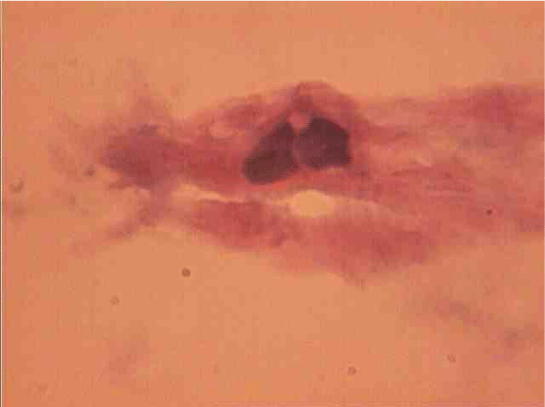
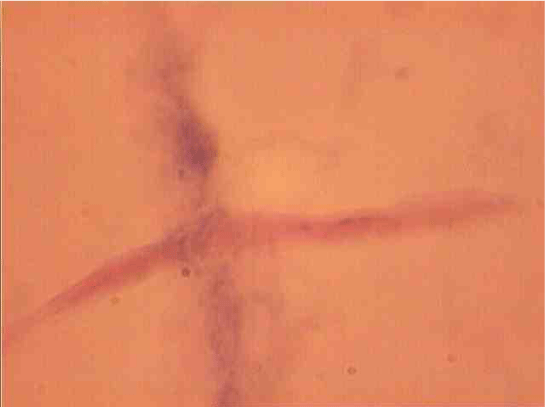
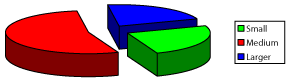
The population of the area of vascular myocytes branching arteries expressed different heteromorphic. Often there are different forms branched SM (Figure 7), with Um tortuous (twisted) nuclei (Figure 8), the peripheral position of the nucleus (Figure 9) .In addition, the share of dual-core four times (to 0.8%) SM. There are muscle cells with micronuclei (Figure 10), dividing by mitosis (Figure 11) and signs of death (Figure 12). In a population of mononuclear myocytes varies the ratio of small (31.4%), medium (40.5%) and high (28.1%) SM. Volumetric detailed pie charts (Figures 13,14) to evaluate the size of SM modulation classes in straight sections and areas of branching renal arteries newborns.
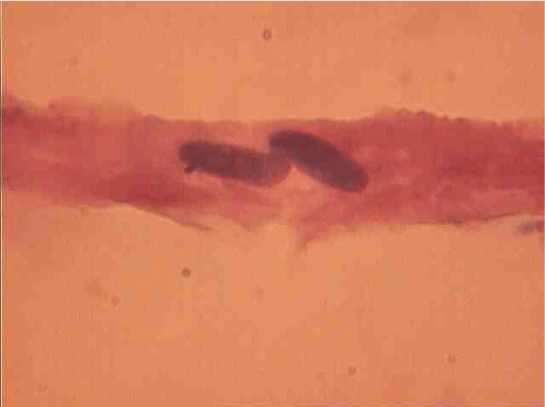
SM dual-characterized, compared to single-core, large size (Figure 15). Thus, their average length is increased to 1.8 (P <0.01), and the cross section - in a 1, 7 (P <0,01) times. Area and volume dual-core cell above, respectively, 4.5 (P <0.01) and 10,4 (P <0,01) fold. This same pattern was observed in the measurement of myocyte nuclei: their length exceeds a mononuclear 1,3 (P <0,01), a cross-section - in 1,4 (P <0,01), and the area and volume of a 2.8 ( P <0,01) and 2,7 (P <0,01) fold.
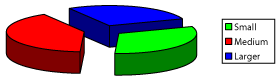
Quantitative analysis of dimensional parameters of dual-core SM shows that in their composition, there are three subpopulations: small (up to 5000 mcm3 9.4% - (Figure 16)), medium (5 000 - 10 000 mcm3 65.3% - (Figure 17)) and large (more than 10,000 mcm3 25.3% - (Figure 18)). The ratio between the bulks represented in subpopulations expanded pie chart (Figure 19).
Dual сore myocytes also differ in their nuclei topography. There SM with nuclei lying one behind the other (Figure 10) or in parallel (Figure 17).
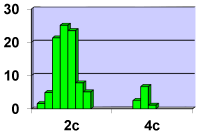
Cytophotometry redistribution study shows ploidy classes in the population of smooth muscle cells in the zone of the branching renal arteries (Figure 20), Table 1).
Discussion of the Data
Unlike straight segments laminar character movement, and field bends flow dividers are special sections with turbulence, swirling, recirculation, and complex shear stress distribution on the nature of the arterial wall [5,8]. It is important to bear in mind that the pressure (stress / strain) and flow (shear) can regulate the function of blood [9], differentiation, proliferation and migration of vascular myocytes [9,10].
The morphological laboratories Yaroslavl Medical University found that areas dividers fetal blood and adult arterial flow distinguished by the presence of additional regulatory smooth complexes in the inner membrane of vessels [11,12]. SM artery walls respond to hemodynamic stress cytoskeleton reorganization and migration [13]. Development in the tunica intima of the SM and the musculo-elastic sphincter is a consequence of the penetration of SM medium choroid into the interior through the Fenestra in the elastic membrane [14]. The role of migration in SM endothelin and platelet-derived growth factor [14,15].
It was established that in the arterial wall area of blood flow dividers marked a pronounced polymorphism of smooth muscle cells, their hypertrophy and polyploidy. In the analysis of mechanisms polyploidy smooth muscle tissue was shown that growth of tangential stress and increased pressure in the vessels associated with activation of DNA synthesis in the nucleus of muscle cells [12, 16]. In this work in areas of branching renal arteries newborns revealed mitotic division of smooth muscle cells, and increasing the number of dual-core form. It is known that various damaging effects lead to an increase in the amount of cell population’s binucleate forms vascular wall [17], it is considered as a variant of polyploidy [18].
In our laboratory we show that the regeneration conditions morphogenesis rat aortic population becomes more reactive SM tunica: labeled nuclei index compared to control them in 53-fold increases in [19]. Thus, both types of SM polyploid with increasing load receptacle (turbulent motion twists and blood recirculation) are formed by polyploidy mitosis. Last according V.Ya. Brodsky & I.V. Uryvaeva [18] is a variant of the conventional mitosis stopped at one stage or another. About the origin of the mitotic dual-core elements is also evidenced by its own data, showing that the relationship between the DNA content in the nuclei of the same dual-core myocyte close to 1.0. DNA replication provides the appearance of new cells or existing polyploidy, ie strengthening of intracellular regeneration processes [20].
In the course of the study shows that the population of the SM renal arteries expressed different heteromorphic. This is especially demonstrated by the presence of small, medium and large single and dual-core vascular myocytes. It is known that SM internal organs (visceral SM) have similar sized subpopulation, where it is attributed to a different functional purpose [21]. The authors believe that a group of small SM is young, actively synthesizing protein cells having proliferative activity; SM’s average form the basis of population and retain proliferative potential; SM is a large terminal elements lose their ability to proliferate.
Thus, these data confirm the information [10,21] about the heterogeneity of the SM populations. Isolated phenotype small phase (fast) SM, capable of passing through hypertrophic growth and tonic (slow) vascular SM undergoing hyperplasia [9]. Load changes can cause phenotypic modulation of myocyte proliferation and SM [9,10]. Thus, hemodynamic conditions can be a factor affecting the properties SM vascular wall. Phenotypic modulation myocytes, which occur in the arteries that are highly branched, anastomosing, many turns, are discussed to initiate the development of hypertension, heart failure, ischemia, obesity and diabetes [22].
- Kamenskaya NL (1955) Еndothelial structure of the renal arteries and veins. Reports of the USSR Academy of Sciences 103: 495-498.
- Reale L, Luciano L (1966) Electronenmicroskopische Beobachtungen an Stellen der Astabgabe der Arterien. Angiologica 3: 226–239. Link: https://goo.gl/HlwLKU
- Thubrikar MJ (2007) vascular mechanics and pathology. NY: Springer Sience + Busines Media 494. Link: https://goo.gl/zpKSfH
- Palombo C, Kozakova M (2016) Arterial stiffnes, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 77: 1–7. Link: https://goo.gl/dHmnxI
- Gansburgskiy AN (1995) The structure of arteries and hemodynamic characteristics in the area of the mouth of the waste receptacles. Morphology: Sankt-Peterburg 108: 82–91.
- Cokelet GR (1980) Reology and hemodynamics. Annu Rev Phisiol 42: 311–324. Link: https://goo.gl/7K2LBW
- Gansburgskiy AN, Yal'tsev AV (2017) Morphology and Ploidy of Smooth Muscle Cells in Chorionic Arteries under Different Hemodynamic Conditions. Bull Exper biol Med 162: 507–510. Link: https://goo.gl/y2kJv7
- Caro CG, Pedley TJ, Schroter RC, Seed WA (1978) The mechanics of the circulation. Oxford: Univ. Press 624. Link: https://goo.gl/vOUxlS
- Fisher SA (2010) Vascular smooth muscle phenotypic diversity and function. Physiol Genomics 42A: 169–187. Link: https://goo.gl/nziXrq
- Hao H, Gabbiani G, Bochaton-Piallat M-L (2003) Arterial Smooth Muscle Cell Heterogeneity Implications for Atherosclerosis and Restenosis Development. Arterioscler Thromb Vasc Biol 23: 1510–1520. Link: https://goo.gl/zbneRF
- Gansburgskii AN, Yal'tsev AV (2015) Features of morphogenesis of blood vessels during fetal placental insufficiency in pregnant women. Russian Bull Perinatology and Pediatrics 60: 45-49.
- Gansburgskiy AN, Yal'tsev AV (2004) Polyploidy smooth muscle cells of the coronary arteries of the heart. Bull Exper biol Med 138: 589–591. Link: https://goo.gl/Mssutg
- Hodebeck M, Scherer C, Wagner AH, Hecker M, Korff T (2014) TonEBP/NFAT5 regulates ACTBL2 expression in biomechanically activated vascular smooth muscle cells. Front Physiol 5: 467. Link: https://goo.gl/zGu6M2
- Jimenes M, Doret D, Choussat A, Bonnet J (2000) Immunohistological and ultrastructural analisis of the intimal thickening in coerelation of human aorta. Cardiovas Res 41: 737–745. Link: https://goo.gl/Er5GFM
- Shehonin BV, Tararak EM, Grjaznov OG, Zotikov AE, AV Pokrovsky (2003) Phenotypes of smooth muscle cells in Takayasu's disease. Arch Pathol: Moscow 65: 31–35. Link: https://goo.gl/bbfZXr
- Kaufman OJ (1987) Smooth muscle tissue. In: Structural basis of adaptation and compensation of disturbed functions: manual. Moscow: Medicine 131–153.
- Gansburgskiy AN, Pavlov AV (1993) Mitotic origin of binucleate cells in the regenerating endothelium. Ontogenez: Moscow 24: 79–82.Link: https://goo.gl/DMQAXI
- Brodsky VYa, Uryvaeva IV (1985) Genome multiplication in growth and development. Cambrige: Univ. Press 305. Link: https://goo.gl/LZIHgo
- Gansburgskiy AN, Pavlov AV (1998) Proliferative cell differons properties of the vascular wall. Morphology: Sankt-Peterburg 113: 66–70. Link: https://goo.gl/VSwgAK
- Sarkisov DS, Pal'tsev MA, Khitrov NK (1997) Total human pathology. Moscow: Medicine 608.
- Zashihin AL, Celine J (2001) visceral smooth muscle tissue. Arkhangelsk: Publishing Center SSMU 195.
- Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol 84: 767–801. Link: https://goo.gl/LmH3fU
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
