Annals of Circulation
Zero-Flow Pressure of the Cerebral Microcirculatory Bed at Concomitant Traumatic Brain Injury
Alex Trofimov1,2*, Michael Dobrzeniecki3, George Kalentyev2, Michail Karelsky1, Andrew Abashkin1, Artem Krasilnikov1 and Svyatoslav Korolev1
2Department of Neurosurgery, Nizhniy Novgorod State Medical Academy, 1, Minin Square, Nizhniy Novgorod, 603950, Russia
3Department of Neurosurgery, Spine Surgery and Interventional Neuroradiology DONAUISAR Klinikum Deggendorf, Perlasberger Str. 41 94469 Deggendorf, Germany
Cite this as
Trofimov A, Dobrzeniecki M, Kalentyev G, Karelsky M, Abashkin A, et al. (2017) Zero-Flow Pressure of the Cerebral Microcirculatory Bed at Concomitant Traumatic Brain Injury. Ann Circ 2(1): 019-023. DOI: 10.17352/ac.000007Zero-flow pressure (ZFP) is an important parameter of a microcirculation. The aim is to determine the status of the ZFP at concomitant traumatic brain injury with and without the development of intracranial hematomas.
Material and Methods: The results of the treatment of 80 patients with severe head injury and polytrauma was studied. M:F - 44:36. Their average age was 34.3 ± 14.5 years (min 15: max 73). Depending on the presence of intracranial hemorrhages the patients were divided into 2 groups. Wakefulness according to Glasgow Coma Score (GCS) was 10.4 ± 2.6 in the 1st group and 10.6 ± 2.8 in the 2nd group. The Injury Severity Score (ISS) was 32 ± 8 in the 1st group and 31 ± 11 in the 2nd group. Epidural hematomas were revealed in the 2nd group in 7 patients, subdural hematomas in 27 persons and multiple hematomas in 4 sufferers. All the sufferers were operated within the first 3 days. During the first day 30 patients (78.9%) were operated.
All the patients were subjected to the transcranial Doppler of the both middle cerebral arteries and the evaluation of the mean arterial pressure. Based on the data obtained the cerebral perfusion pressure and the ZFP were calculated. The comparisons between the groups were performed by using the Student’s t-criterion.
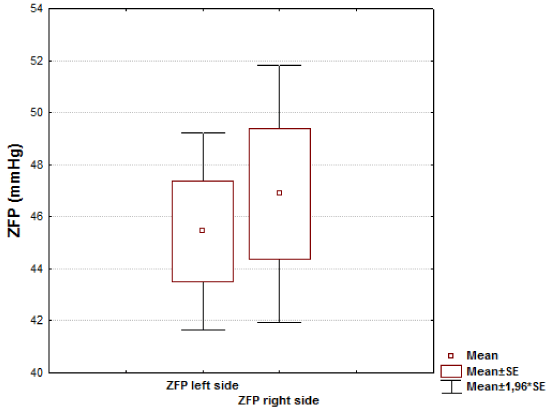
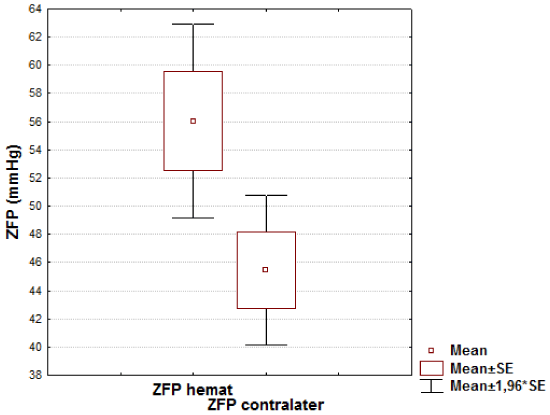
Results: There was no significant difference in ZFP between the left side and the right side in 1st group (46.88±14.05 mmHg vs. 45.44±10.73 mmHg, respectively, t=0.45; р=0.65) The average ZFP values in each of the groups (with or without hematomas) appeared to be statistically significantly higher than a mean value in control group (32,9 ± 3,6 mmHg, р<0.01). The intergroup comparison of the ZFP showed a statistically reliable increase in its level in the 2nd group on the side of the removed hematoma as compared with opposite side (56.02±21.68 mmHg vs. 45.43±16.71 mmHg, respectively, t=2.38; р=0.019p=0,019).
Abbreviations
APsys: Systolic Arterial Pressure; APdia: Diastolic Arterial Pressure; GCS: Glasgow Coma Score; GOS: Glasgow Outcome Scale; CPP: Cerebral Perfusion Pressure; CTBI: Concomitant Traumatic Brain Injury; ICH: Intracranial Hematomas; ICP: Intracranial Pressure; ISS: Injury Severity Score; SD: Standard deviation; Vdia: Diastolic cerebral blood flow velocity; Vsys: Systolic cerebral blood flow velocity; WT: Arterial-arteriolar smooth-muscle wall tone; ZFP: Zero-Flow Pressure
Introduction
The maintaining of sufficient pressure is the most important aspect of the prompt and adequate therapy of traumatic brain injury (TBI) [1].
The cerebral perfusion pressure (CPP) is currently defined as the difference between the mean arterial and intracranial pressure (ICP) [2].
At the same time, the availability of pressure gradients developing in the brain at severe TBI makes the CPP value to be rather averaged and, thus, not reflecting a “real” cerebral perfusion [3].
It is suggested that the cerebral microcirculation is more precisely reflected by the “effective” perfusion pressure, which is defined as the difference between the mean arterial pressure and the pressure below which the local pial blood pressure is inadequate to prevent cessation of blood flow. This parameter was called the “zero-flow” pressure (ZFP) or critical closing pressure [4,5].
The ZPF-model was proposed in 1951 by A. Burton [6] and was later developed by R. Dewey и S. Permutt [7,8].
Their studies showed that the ZFP is the sum of the cerebral parenchymal pressure, the pressure in venous sinus of the dura mater and the pressure of the arterial- arteriolar smooth-muscle wall tone. It was further determined that the first two parameters constitute the value of ICP.
Thus, the ZFP is determined by the following formula [4]:
ZFP=ICP+WT,
ZFP – zero-flow pressure,
ICP – intracranial pressure,
WT – arterial-arteriolar smooth-muscle wall tone.
ZFP is a valuable and clinically relevant tool in cerebrovascular research, as it allows to estimate changes in cerebrovascular tone and minimal cerebral perfusion pressure to prevent collapse of vessels and cerebral ischemia.
A modern approach to the ZFP calculation is based on the assessment of the cerebrovascular impedance state [5] and provides for the simultaneous evaluation of a number of secondary parameters for physical characteristics of the brain. This method is the most precise one, since when using thereof the ZFP values may only be positive and that complies with the original concept of A. Burton [6].
The ZFP value is of physiological importance and considered as a result of smoothing pulse fluctuations in arterial pressure to a level thereafter the avalanche-like collapsing of the microcirculatory bed occurs [9].
It was shown that the CPP and ICP values reliably correlate with the ZFP values. Thus, the ZFP determination becomes practically important since it enables to perform the noninvasive assessment of the cerebral perfusion state when its invasive assessment by the direct intracranial pressure measurement is impossible [5].
Since then, ZFP has been studied in various microvascular beds under various conditions (premature infants [4], hydrocephalus [10], cerebral vasospasm [11] etc).
It has been shown that with a moderate intracranial hypertension in cases of traumatic brain injury, the ZFP value is on the average 40.36±17.44 mmHg but at the “plateau-wave” development it increases by almost 40% to 54.71±17.66 mmHg [12].
However, the ZFP state in the cerebral microcirculatory bed of patients with concomitant TBI and traumatic intracranial hematomas as one of the most severe and widespread form of the brain damage still remains currently understudied [5,13].
The purpose of the study is to determine the ZFP state in patients with severe CTB injury upon the development of intracranial hematomas.
Materials and Methods
Study
A retrospective observational study was performed at the Trauma Center Level I of a tertiary university hospital in Russia. All experiments were in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study was approved by the Ethics Committee of Nizhny Novgorod Regional Clinical Hospital named after N.A. Semashko. All the patients gave informed consent to participate in the study.
Population
We studied 80 patients with severe CTBI who were treated at the Nizhny Novgorod Regional Clinical Hospital named after N.A. Semashko in 2013–2016. The study involved 44 males and 36 females. The patients were aged 15 to 73 years, mean age being 34.3±14.5 years. All of them received therapy according to the international protocol Advanced Trauma Life Support.
Depending on the presence of intracranial hemorrhage, the patients were divided into two groups: the group 1 included 42 CTBI patients without ICH, the group 2 comprised 38 sufferers with CTBI and cerebral compression by ICH. The groups were comparable in age and severity of TBI and concomitant lesions.
The severity of the condition according to the Glasgow Coma Scale in the group 1 averaged 10.4±2.6, in the group 2 it was 10.6±2.8.
The severity of injuries according to ISS scale (Injury Severity Score) in the group 1 averaged 32±8 and was 31±11 in the group 2.
Among 38 patients of the group 2 epidural hematomas were revealed in 7 persons, subdural ones were found in 27, multiple hematomas were revealed in 4 sufferers. All hematomas were located mainly in the fronto-temporal region. All the sufferers underwent surgery within the first three days. Thirty patients (78.9%) were operated during the first day.
The treatment outcomes were assessed according to Glasgow Outcome Scale (GOS) on discharge from hospital (Table 1).
Simultaneously the blood pressure (IntelliView MP5, Philips Medizin Systeme, and Germany) was assessed and the transcranial Doppler examination was performed, thus, ensuring equitype conditions for the cerebral blood flow study.
Cerebral blood flow velocity in the middle cerebral artery was measured by transcranial Doppler through the temporal window with a 2-Mhz probe (Sonomed 300M, Spectromed, Russia) according to the method developed by Aaslid. The probe was positioned over the temporal bone window above the zygomatic arch and fixed. This procedure ensured that the angle and individual depth of insonation remained constant during investigation. The temporal ultrasound window and depth of the insonation giving the highest velocities were used for all measurements. Two investigators performed all measurements (A.T. and G.K.). Recordings were made with subjects in supine position, the head elevated to 300. A minimum of 10 min time windows of CBFV, heart rate and mean arterial pressure (MAP) were simultaneously recorded on a computer and stored with a sample rate of 50 Hz by an A/D converter (AX -21, Nizhny Novgorod, Russia) [13]. During the measurements, PaO2, PaCO2 and body temperature were within normal ranges and patients were normotensive. All patients during the study were with spontaneous breathing, required neither sedation nor pharmacological support for the blood pressure.
Exclusion criteria for all patients were an irregular heart rhythm, insufficient temporal bone window, atonic coma, refractory traumatic shock or a life expectancy <24 h.
ZFP: For the calculation of the zero-flow pressure (critical closing pressure) of the cerebral microcirculatory bed we used the formula proposed by Aaslid and Ogoh [14,15]:
where ZFP- zero-flow pressure (mmHg),
APsys – systolic arterial pressure (mmHg),
APdia – diastolic arterial pressure (mmHg),
Vsys – systolic cerebral blood flow velocity (cm/sec),
Vdia – diastolic cerebral blood flow velocity (cm/sec).
Statistical analysis
The obtained data had normal distribution, so they were presented as “mean ± SD”. Comparison between the groups was carried out according to Student’s t-test. Figures also show minimum and maximum (whiskers) values. The level of significance was taken at p<0.05. The results were analyzed using Statistica 7.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Analysis of the studied parameters in the groups showed that the mean ZFP values in each group of sufferers with severe CTBI (both with and without hematomas) appeared to be statistically significantly higher than a mean value in control group (32,9 ± 3,6 mmHg, р<0.01).
There was no significant difference in ZFP between the left side and the right side in 1st group (46.88±14.05 mmHg vs. 45.44±10.73 mmHg, respectively, t=0.45; р=0.65) (Figure 1).
The most significant differences were revealed in patients of the group 2: the mean ZFP in the perifocal zone of removed hematoma remained significantly higher compared to that in the symmetrical zone of the contralateral hemisphere (56.02±21.68 mmHg vs. 45.43±16.71 mmHg, respectively, t=2.38; р=0.019) (Figure 2).
The intergroup comparison showed no statistically significant differences between the mean ZFP values in patients of the group 2 on the side opposite to the removed hematoma as compared to the group 1 (р>0.05).
At the same time in the perifocal zone of the removed ICH the mean ZFP values were statistically significant higher than in CTBI patients without any developed hematomas (р=0.015 and р=0.048).
Analysis of ZFP values in various types of intracranial hematomas showed no statistically significant differences (р>0.05).
Also, no significant effects of patient age on the ZFP value were found (р > 0.05)
Discussion
The disorders of the cerebral microcirculation play a key role in the development of the brain hypoperfusion and secondary ischemia episodes in case of any brain damages [16].
One of the numerous parameters, which describe the state of the cerebral microcirculatory bed, is the zero-flow pressure [17].
In wide sense, the cerebral ZFP plays an important role in limiting the excessive perfusion of the brain tissue, which damages the structure of the blood-brain barrier and perhaps the glymphatic system [18].
We have shown that the ZFP in CTBI patients significantly exceeds normal parameters (p<0.001).
In our opinion, one of the possible causes for this excessive value may be the increasing concentration of catecholamines accompanying the acute stage of CTBI [19].
Another reason for the increasing ZFP may be the rise in the intrathoracic pressure in case of a pulmonary injury, which was found in our study in all patients. We have not carried out the intrathoracic pressure monitoring but there is an opinion that a lung injury significantly increases ZFP [20].
We have shown that the ZFP statistically significant differs in the groups of CTBI patients with the brain compression by ICH and without ICH. The comparability of the groups in respect of the concomitant injury structure proves that the revealed ZFP changes obviously result from the traumatic compression of the brain.
The statistically significant ZFP increase (р=0.019) on the side of the removed ICH has apparently a complicated genesis.
One of the causes may be the microvascular vasospasm of pial vessels in the perifocal zone [12]. It should be note that the transcranial ultrasonic Doppler examination in contrast to the laser Doppler flowmetry does not enable to assess the spasm of the vessels constituting the microvascular bed and that was first limitation of our work.
Second limitation was suggest that, proximal vasospasm causes turbulence in cerebral blood flow, which will impair the linear relationship between pressure and cerebral blood flow velocity, thus leading to an underestimation of ZFP.
On the other hand, although the intracranial pressure monitoring in patients of the both groups was not performed during the study, the control CT scans did not identify any shift of septum pellucidum more than by 5 mm in any of the patients.
So, it may imply the absence of any significant interhemispheric gradients of ICP and hence, (according to the ZFP formula), with the constant ICP - the increase in the vascular wall tone (WT) [21] as the cause for the increasing ZFP in the perifocal zone [22].
Thus, based on the results of our study we may draw the conclusion that at the early stage of a severe concomitant TBI there are marked changes in ZFP and cerebral microcirculation, which are exacerbated by the development of ICH.
On the practical level the ZFP assessment in patients with brain injuries may be the basis for the noninvasive determination of the “effective” CPP value [23], for the prompt detection and adequate treatment of secondary cerebral insults.
We believe that the results of our study shall provide conditions for a differentiated approach to solving the question on timing of orthopedic correction of extracranial injuries in polytraumazed patients - during the period of microcirculatory normalization and the minimal risk of the secondary brain injury.
Conclusion
Zero-flow pressure in patients with combined traumatic brain injury is significantly increased compared to the normal value. After evacuation of hematoma in the former perifocal zone zero-flow pressure remains significantly elevated compared to the symmetrical zone in the contralateral hemisphere.
- Lewis PM, Smielewski P, Rosenfeld JV, Pickard JD, Czosnyka M (2012) Monitoring of the association between cerebral blood flow velocity and intracranial pressure. Acta Neurochir Suppl 114: 147–152. Link: https://goo.gl/31d2JB
- Rosner M, Rosner S, Johnson A (1995) Cerebral perfusion pressure Management protocol and clinical results. J of Neurosurg 83: 949–962. Link: https://goo.gl/hRKj0k
- Sahuquillo J, Poca MA, Arribas M, Garnacho A, Rubio E (1999) Interhemispheric supratentorial intracranial pressure gradients in head-injured patients: are they clinically important? J of Neurosurg 90: 16-26. Link: https://goo.gl/qbdmHK
- Rhee C, Fraser C, Kibler K, Easley RB, Andropoulos DB, et al. (2014) The ontogeny of cerebrovascular pressure autoregulation in premature infants. J of Perinatolology 34: 926–931. Link: https://goo.gl/348RSl
- Varsos GV, Budohoski KP, Kolias AG, Liu X, Smielewski P, et al. (2014) Relationship of vascular wall tension and autoregulation following traumatic brain injury. Neurocritical Care 21: 266–274. Link: https://goo.gl/NU9Hc0
- Burton A (1951) On the physical equilibrium of small blood vessels. Am J of Physiology 164: 319–329. Link: https://goo.gl/FIhCdo
- Dewey RC, Pieper HP, Hunt WE (1974) Experimental cerebral hemodynamics-vasomotor tone, critical closing pressure, and vascular bed resistance. J of Neurosurgery 41: 597-606. Link: https://goo.gl/RUPcf9
- Permutt S, Riley R (1963) Hemodynamics of collapsible vessels with tone. The vascular waterfall. J Appl Physiol 18: 924–932. Link: https://goo.gl/Gm3I9Z
- Varsos G, Richards H, Kasprowicz M, Reinhard M, Smielewski P, et al. (2014) Cessation of Diastolic Cerebral Blood Flow Velocity: The Role of Critical Closing Pressure. Neurocritical Care 20: 40-48. Link: https://goo.gl/1PFbMI
- Varsos G, Czosnyka M, Smielewski P, Garnett MR, Liu X, et al. (2015) Cerebral critical closing pressure in hydrocephalus patients undertaking infusion tests. Neurological Research 37: 674-82. Link: https://goo.gl/2aepwL
- Soehle M, Czosnyka M, Pickard J (2004) Critical Closing Pressure in Subarachnoid Hemorrhage Effect of Cerebral Vasospasm and Limitations of a Transcranial Doppler-Derived Estimation. Stroke 35: 1393-1398. Link: https://goo.gl/F4EHXF
- Varsos G, de Riva N, Smielewski P, Pickard JD, Brady KM, et al. (2013) Critical Closing Pressure during Intracranial Pressure Plateau Waves. Neurocritical Care 18: 341-348. Link: https://goo.gl/3mLbsp
- Trofimov A, Kalentiev G, Gribkov A, Voennov O, Grigoryeva V (2015) Cerebrovascular Time Constant in Patients with Head Injury. Acta Neurochir Suppl 121: 295-299. Link: https://goo.gl/8C10La
- Aaslid R (1992) Cerebral hemodynamics, Transcranial Doppler. Edited by Newell D, Aaslid R New York: Raven Press, USA. Link: https://goo.gl/4BuoNG
- Ogoh S, Fisher J, Young CN, Fadel PJ (2011) Impact of age on critical closing pressure of the cerebral circulation during dynamic exercise in humans. Exp Physiol 96: 417–425. Link: https://goo.gl/GWuARS
- Laan Mark ter (2014) Neuromodulation of cerebral blood flow. PhD thesis Groningen University The Netherlands, Neuroscience Department. Link: https://goo.gl/QwsRqo
- Czosnyka M, Smielewski P, Piechnik S, Al-Rawi PG, Kirkpatrick PJ, et al. (1999) Critical closing pressure in cerebrovascular circulation. J Neurol Neurosurg Psychiatry 66: 606–611. Link: https://goo.gl/msa6P7
- Plog B, Dashnaw M, Hitomi E, Peng W, Liao Y, et al. (2015) Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 35: 518-526. Link: https://goo.gl/qb6lE6
- Heistad DD, Marcus ML, Gross PM (1978) Effects of sympathetic nerves on cerebral vessels in dog, cat, and monkey. Am J Physiol 235: 544–552. Link: https://goo.gl/ft91CA
- Dawson S, Panerai R, Potter J (1999) Critical closing pressure explains cerebral hemodynamics during the Valsalva maneuver J Appl Physiol 86: 675–80. Link: https://goo.gl/pPORao
- Richards H, Czosnyka M, Pickard J, (1999) Assessment of critical closing pressure in the cerebral circulation as measure of cerebrovascular tone. Acta Neurochirgica 141: 1221–1227. Link: https://goo.gl/sPKzNG
- Michel E, Hillebrand S, vonTwickel J, Zernikow B, Jorch G (1997) Frequency dependence of cerebrovascular impedance in preterm neonates: A different view on critical closing pressure. J Cereb Blood Flow Metab 17: 1127–1131. Link: https://goo.gl/zRnraF
- Thees C1, Scholz M, Schaller M D C, Gass A, Pavlidis C et al. (2002) Relationship between Intracranial Pressure and Critical Closing Pressure in Patients with Neurotrauma. Anesthesiology 96: 595–599. Link: https://goo.gl/Bxi00u
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
