Annals of Circulation
Vasodilatory Effect of the Dissolved Glycine locally applied on Pial Microvessels
Tyukina ESa*, Sheshegova EV1a, Nartsissov YR2a, Podoprigora GI3a
2PhD in Physical and Mathematical Sciences,Associate Professor, Russia
3Doctor of Medical Sciences, Professor
aInstitute of cytochemistry and molecular pharmacology, 6th Radialnaya str. 24/1, Moscow, Russia
Cite this as
Tyukina ES, Sheshegova EV, Nartsissov YR, Podoprigora GI (2017) Vasodilatory Effect of the Dissolved Glycine locally applied on Pial Microvessels. Ann Circ 2(2): 034-037. DOI: 10.17352/ac.000010By the method of biomicroscopy it was shown that a single application of a dissolved glycine on the parietal region of the rat brain (“open window” technique) leads to a vasodilatation - an increase in arteriolar diameter about 1.5-2 times. There were no changes in the microcirculation when saline applied under similar conditions. It was also shown that there were no pH effects on the microvessels. For the testing of the vasodilatory effect of glycine in arteriol spastic conditions the adrenomimetic phenylephrine hydrochloride possessing the vasoconstrictor properties has been used as an agent for ischemic stroke modeling. Further work on the development of an adequate ischemic stroke models is the prospective task for the testing of antiischemic preparations.
Introduction
In recent years, the increasing prevalence of ischemic diseases affecting the vitally important organs especially those of cardiovascular and central nervous system is observed. Ischemic brain injury is accompanied by the severe neurological disorders, such as cognitive deterioration, dysfunction of motor, verbal and other CNS functions. Stroke ranks first among the causes of persistent disability. Correcting the imbalance of excitatory and inhibitory neurotransmitter systems is one of the most promising areas of neuroprotection and therapeutic target. Attention of researchers is attracted to a role of inhibitory neurotransmitter glycine in the mechanisms of acute cerebral ischemia [1]. The widespread use of the drug “Glycine” in patients with neurological disorders, as well as clinical observation of the positive therapeutic effect of the drug in ischemic stroke [2,3], suggest the existence of one or more fundamental biological mechanisms responsible for the neuroprotective effect of the amino acid at the molecular level. Since one of the important pathogenic mechanisms leading to the development of an ischemic stroke is a disturbance of blood supply to the brain, the effect of any drug that is expected to have clinical benefit should be assessed in terms of possible normalization of microcirculation. One of the tests used for an assessment of Glycine direct vasodilatory effect is an intravital biomicroscopy of pial microvessels in a brain of laboratory rats. The first studies demonstrated microcirculatory effect of glycine solution on mesenteric microvessels and on pial arterioles in laboratory rats [4-6]. A capacity of glycine to restore impaired microcirculation in the arterioles of the rat mesenterium under histamine induced inflammatory alterations was shown revealing it’s great, but little studied yet, antiinflammatory potential [5]. Effect of glycine on pial arterioles has been performed by using the original direct biomicroscopy with “open window” technique [6]. The significant vasodilatory effect of glycine on microcirculation in pial microvessels in the cortex of a parietal area of the rat brain was shown. The aim of present work was to study if there is any dependence of Glycine effect on the pH values and the arteriole (blood microvessels) diameter size. The same parameters were used in control study with saline. Also, the possibilities of using the α- adrenomimetic phenylephrine hydrochloride preparation possessing the vasoconstrictory properties has been tested as a potential model of ischemic stroke and for an assessment of vasodilatory effect of the amino acid glycine in the conditions of experimentally induced vasospasm.
Methodology
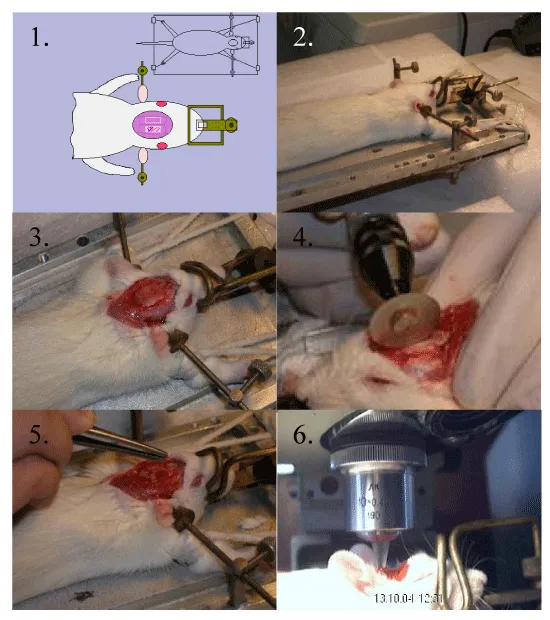
Experiments were performed on 35 male Wistar rats weighting 200-350 g. The animals were intraperitoneally anesthetized with 400 mg/kg chloral hydrate. Glycine (1 M) was dissolved in physiological saline, Glycine (1 M) with given pH (equal to 2, 3, 4, 8, 9, 10), saline solutions with given pH (equal to 2, 3, 4, 8, 9, 9.7), solutions of phenylephrine hydrochloride in concentrations of 0.015 mM, 0.03 mM, 0.06 mM, 0.3 mM, 0.6 mM, 0.9 mM were used in this research. All the solutions (kept in water bath under 37ºC) were applied to the surface of the brain parietal area (0.1 mL) after craniotomy and the membrane removing (“open window technique”). The stages of this technique are presented (Figure 1). Permanent control of all the solutions tested at temperature 37ºC (water bath). Control animals were treated with physiological saline. Direct visual monitoring of the microcirculatory bed (Fig. 1.6) was performed using a digital microscope camera DMC 300. Images of microvessels were obtained with a surface-contact long-focus objective (x10). The method allowed us to obtain images at a 100fold magnification. The state of microcirculation in the pial arterioles of the brain cortex was estimated by the measuring of diameters of arterioles (20 – 200 μm) in the program Adobe Photoshop CS5. This parameter used as a major characteristic of blood flow in the microvessels (Figure 1).
Results and Discussion
The study performed revealed significant vasodilatory activity of glycine solution applied to the surface of the pial microvessels. These results were in full compliance with early reported data [6]. At the same time the mechanisms of the glycine vasodilatory influence are still poor understood. Dacey Jr. [7], showed that the isolated rat cerebral blood vessels may respond to changes in the pH of saline solution in which they are placed.
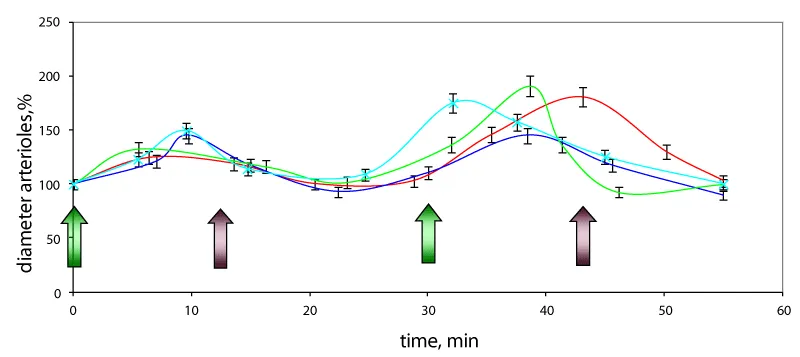
Our study showed no dependence on pH value of saline solution (in the range of 2 to 9.7) applied to the surface of the cerebral membrane. No change of the arteriole’ diameters due to this fact was observed. Thus, the diameter of the pial arterioles doesn’t depend on the pH value of the locally applied solution. Vascular reaction was observed 1 – 3 min after application of glycine (Figure 2). The pronounced dilation of the arterioles has been registered. Their diameter increased by 150 – 250%. The diameter of dilated microvessels returned to an initial size 10 – 15 min after application of glycine. The diameter of venules at the same conditions remained unchanged. Also, the influence of glycine solution with the different pH in the range of 2 to 10 on the diameter of blood microvessels was investigated. The changes were the same as in the previous experiments, regardless of the pH of the solution. (Figure. 2).
To study the action of the amino acid glycine in vasospastic conditions we tested phenylephrine hydrochloride the synthetic adrenomimetic drug stimulating the α-adrenergic receptors with a little effect on β-receptors of the heart. It causes constriction of arterioles and increases blood pressure. According to Shigehiko Ogoh et al. [8], when administered intravenously to healthy adult, phenylephrine hydrochloride was reported to induce vasoconstriction of the arteries of the brain.
The series of experiments for selection of the concentration of phenylephrine hydrochloride have been performed aiming to obtain the maximum arteriolar spasm. For this purpose the solutions phenylephrine hydrochloride with concentrations of 0.015 mM, 0.03 mM, 0.06 mM, 0.3 mM, 0.6 mM and 0.9 mM have been tested.
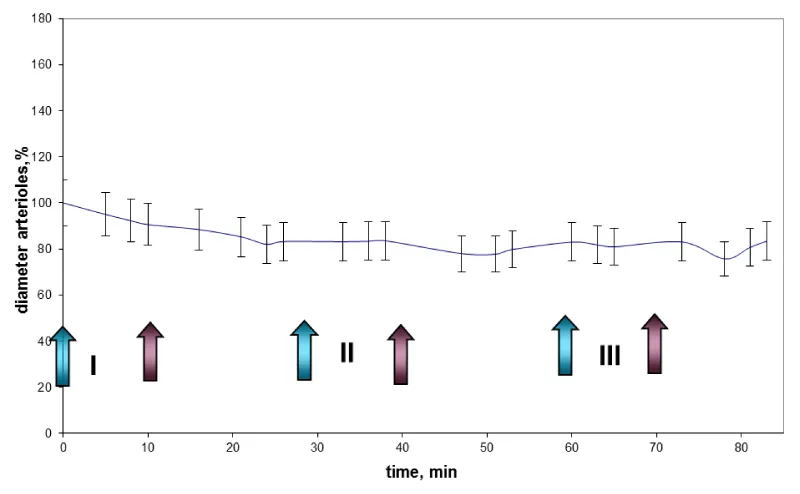
It was shown that the phenylephrine hydrochloride solution at low concentrations didn’t lead to significant reduction in the diameter of arterioles (Figure 3). The effect was not expressed enough to be used as a model for ischemic stroke. Moreover, an application of the solution of phenylephrine hydrochloride with higher concentrations even caused an increase in arteriolar diameters in 1.5 – 2 times (Figure 4). Just after flushing the reagent with saline some reduction of the arteriole diameter in 1.5 – 2 times was observed. It was comparable to the initial state of the vessels used as a model of vasoconstriction of the cerebral blood vessels (Figures 3,4).
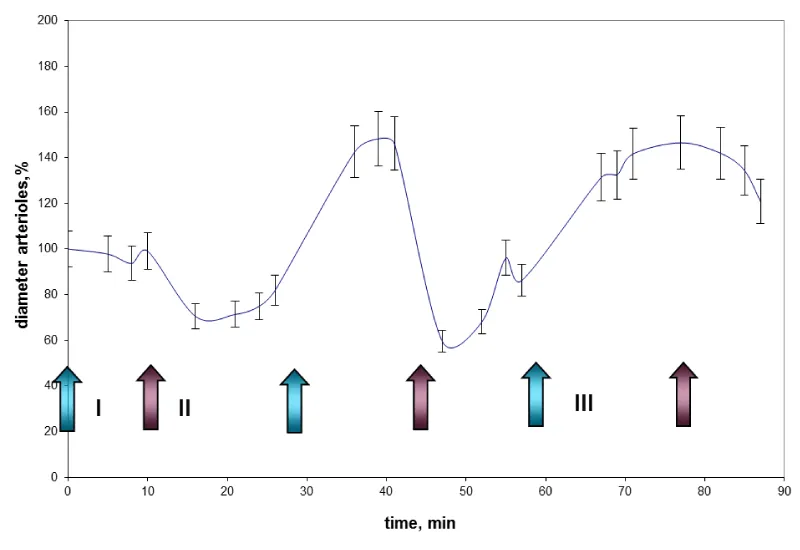
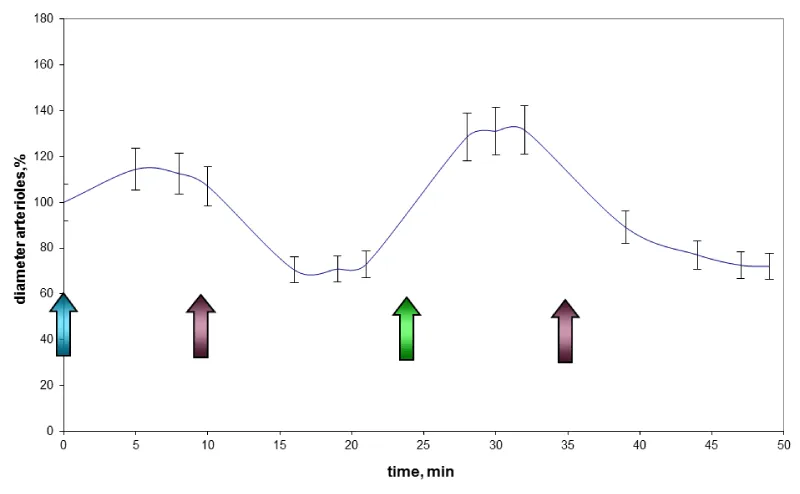
An experiment was carried out to find out the influence of a 1 M solution of the amino acid glycine on the diameter of pre-spastic blood vessels. During this experiment, solution of phenylephrine hydrochloride with concentration of 0.3 mM was applied to the surface of the rat brain; this solution was maintained for 10 minutes, and then flushed with saline. After 15 minutes, glycine solution was applied and then, after 10 minutes it was also flushed. The results are shown in figure 5.
As the graph shows, the application of the solution of phenylephrine hydrochloride on the brain surface with a concentration of 0.3 mM increases arteriolar diameter by 10 – 20%. After flushing with saline the arteriolar lumen decreases by 30 - 40% compared to the initial rate. After applying the solution of glycine vasodilatation 30 – 40% more than baseline was observed. Thus, the data obtained indicate the vasodilatory effect of glycine on microvascular spasm.
An overview of the literature available shows that mechanisms of glycine action are not fully understood and contains quite contradictory results. It concerns both glycine molecular signaling activity and its physiological effects in in vivo experiments. The former group of investigation the bioinformatic analysis indicates the key role of NMDA receptors and they’re the most potent subunits NR2A and NR2B in mechanisms of glycine action. The localization of NMDA receptors plays also an important role their function. Synaptic (predominantly NR2A-containing) and extrasynaptic (predominantly NR2Bcontaining) NMDARs have differential functions, promoting survival and death, respectively [9].
The distinct mechanisms may be an explanation of some inconsistence in the data obtained in physiological experiments in vivo. The dose dependence of glycine effect was shown. The positive neuroprotective effect was seen at high dose of the preparation (800 mg/kg) and no effect of the middle dose (400 mg/kg), whereas small doses (80 mg/kg) led to an opposite effect, linked with the damage in nervous system and related apoptotic changes [9]. Talking about physiological level of research since the first published observation [6], our attention was attracted to microcirculation and the role of its disturbances in the pathogenesis of ischemic stroke. Alteration in a balance between synaptic and extrasynaptic NMDA receptors is one of the factors [10].
According to the literature data the glycine plays a multiface role and the mechanisms of its action are distinctive depending on the level of the body organization (molecular, cellular, systemic etc.).
The different results depending on the localization of NMDA and glycine receptors (synaptic or extrasynaptic) may explain its distinct effect (such as neuroprotective activity) and the contradiction in the results reported. For example stimulation of these differently located NMDA receptors can result in opposite effect (beneficial) for synaptical and (negative) for extrasynaptical ones.
On the other hand possibility of diffusion of higher dose of extracellular glycine and vasoactive substances (NO, vasoactive cytokines and peptides) capable to penetrate to neighbor tissues including smooth muscles of the microvessels may be the agents stimulating microcirculation and increasing blood supply in ischemic tissues.
An activation of ionotropic glutamatergic receptors in neurons leads to increase intracellular influx of Ca2+ followed by activation of metabolic pathway signaling the production of vasoactive agents able to penetrate into microvessel smooth musculature inducing dilatory effect in cerebral arterioles. Glutamate and its synthetic analogues N-methyl-D-aspartate (NMDA), kainate, and alphaamino-3- hydroxy-5-methylisoxazole-4-propionate (AMPA) are potent dilator agents in the cerebral circulation. The potential vasoactive agents involved are NOS and NO, CO, adenosine, P-450 monooxygenase products, etc.
A close linkage between concept of tight coupling between increased neural activity and cerebral blood flow is well known. However, mechanisms involved in promoting cerebral vasodilator responses to glutamatergic agents are controversial [11].
An application of direct intravital technique is a reliable approach for testing of integral effect of the tested drug acting with the different mechanisms involved. The possibilities of the visualization have an advantage of the monitoring permit the time and the size of focal ischemic injury measurement that is especially important for the penumbra determination and an assessment of potential therapeutic activity of the drug tested. In this direction the using of the tracers for PET investigation (microPET for laboratory animals) is an important addition for a study of a cerebral microcirculation research and testing of experimental therapy on the laboratory animal models of ischemic stroke.
Of especial importance is the adequate models for reproduction of microcirculatory disturbances linked with brain tissue ischemia and stroke for testing of candidate preparation for its potential microcirculatory activity. The technique of ligation of carotid arterias (one side or bilateral) did not give a reproducible results concerning pronounced alteration in pial microcirculation according to other investigators including our experience. The most reliable technique recommended is [12]. However, the method appeared to be a difficult enough for routine reproducing. Selection of an adequate biomodels for ischemic stroke along with visualization of focal for experimental therapy purposes is of prospective task of our research.
Conclusions
Nowadays it is important to search for new potential substances for the treatment of ischemic stroke and preclinical models for its investigation.
The study performed revealed significant vasodilatory activity of glycine solution applied to the surface of the pial microvessels, and it is correlated with early reported data [4,6]. But previously some conditions that could distort the results of the study were not taken into account. Thus, the study of the effect of saline with pH values from 2.0 to 9.7 on microcirculation in pial microvessels of the rat’s brain showed that there were no pH effects on microvessels. Also, this study allowed us to obtain new data about the effect of glycine on microcirculation in the spastic microvessels of the brain. Its application was followed by pronounced dilatation of arterioles (Figure 2), their diameter increased by 150 –200%. Effect was observed 1 – 3 minutes after application of the amino acid solution. The diameter of microvessels returned to normal 10 – 15 min after application of glycine. Repetitive application of glycine led to a similar increase in the diameter of blood microvessels. This effect remains when glycine was applied to spastic arterioles. Further development and application of the laboratory biomodel of local cerebral ischemia based on chemically or physically induced vasoconstrictory effect followed by microcirculation alteration may be one of the prospective therapeutic targets and value for preclinical testing and evaluation of new microcirculatory active drugs.
- Gusev EI, Skvortsova VI, Dambinova SA, Raevskiy KS, Alekseev AA, et al. (2000) Neuroprotective effects of glycine for therapy of acute ischemic stroke. Cerebrovasc Dis 10: 49-60. Link: https://goo.gl/PbXnx1
- Patent of Russia? 282 398 1992/1997.
- Gusev EI, Skvortsova VI (2001) Cerebral ischemia? Moscow. Link:
- Podoprigora GI, Blagosklonov O, Angoué O, Boulahdour H, Nartsissov YR (2012) Assessment of Microcirculatory Effects of Glycine by Intravital Microscopy in Rats. Conf Proc IEEE Eng Med Biol Soc 2012: 2651-2654. Link: https://goo.gl/GgEkgh
- Podoprigora GI, Nartsissov YR (2009) Effect of glycine on the microcirculation in rat mesenteric vessels. Bull Exp Biol Med 147: 308-311. Link: https://goo.gl/QnBhVq
- Podoprigora GI, Nartsissov YR, Aleksandrov PN (2004) Effect of glycine on microcirculation in pial vessels of rat brain. Bulletin of experimental biology and medicine 139: 675-677. Link: https://goo.gl/CnfQ3d
- Dacey RG Jr, Takayasu M (1989) Effects of inhibitory and excitatory amino acid neurotransmitters on isolated cerebral parenchymal arterioles. Brain Research 482: 393-396. Link: https://goo.gl/qpEcqD
- Ogoh S, Sato K, Fisher JP, Seifert T, Overgaard M, et al. (2011) The effect of phenylephrine on arterial and venous cerebral blood flow in healthy subjects. Clin Physiol Funct Imaging. 31: 445-451. Link: https://goo.gl/DCKUTW
- Yao W, Ji F, Chen Z, Zhang N, Ren SQ, et al. (2012) Glycine exerts dual roles in ischemic injury through distinct mechanisms. Stroke 43: 2212-2220. Link: https://goo.gl/Q8pn1v
- Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11: 682-696. Link: https://goo.gl/ZLRdgZ
- Busija DW, Bari F, Domoki F, Louis T (2007) Mechanisms Involved in the Cerebrovascular Dilator Effects of N- methyl- D-aspartate in Cerebral Cortex. Brain Res Rev 56: 89-100. Link: https://goo.gl/yRkZ8f
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, et al. (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17: 472-476. Link: https://goo.gl/bZLDUA
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
