Annals of Circulation
Coupled blood pressure dynamics in magisterial and small arteries networks and its stabilizing effect on heart functioning within the framework of computer model
Alexander Shmid1, Novopashin MA1 and Andrey Berezin2*
2Researcher of the Company EC-leasing, Moscow, Russia
Cite this as
Shmid A, Novopashin MA, Berezin A (2018) Coupled blood pressure dynamics in magisterial and small arteries networks and its stabilizing effect on heart functioning within the framework of computer model. Ann Circ 3(1): 004-007. DOI: 10.17352/ac.000012Iron has many special functions in the body. More than 65 percent of the body’s iron is in the blood in the form of hemoglobin, which is a protein in red blood cells that transports oxygen to tissues in the body. A compound that carries oxygen to the muscle cells called myoglobin, also requires iron. In addition, iron has a role in many chemical reactions within the body that generate energy. A human body can store excess iron as a reserve. The World Health Organization reported that iron deficiency anemia is one of the most widespread nutrient deficiencies in the globe. Diverse factors may affect its absorption like low dietary intake of iron, deprived iron absorption, or too much blood loss. Moreover, polyphenolic compounds widely found in coffee and tea such as chlorogenic acids, monomeric flavonoids, and polyphenol polymerization products also strongly inhibit dietary nonheme-iron absorption. Children, adolescents, pregnant women, women of child-bearing age, athletes, and older adults are groups at greater risk for iron deficiency. The objective of this review paper is to review factors influencing iron absorption.
Introduction
Minerals are indispensable part of a complete diet of animals. Minerals are inorganic elements that are found in all body tissues and fluids. Even though they yield no energy, they have vital roles in many activities in the body [1,2]. These compounds are necessary for the maintenance of certain physicochemical processes which are essential to life because they are chemical constituents used by the body in many ways. Every form of living matter requires these inorganic elements or minerals for their normal life processes [3,4].
The value of micro-minerals in human, animal and plant nutrition has been well recognized [5,6]. Deficiencies or disturbances in the nutrition of an animal cause a variety of diseases and can arise in several ways. Iron is the most abundant element on earth, yet only trace elements are present in living cells. Iron is essential to all cells of the human body. Iron is a micro-mineral that has a number of key functions. It’s a major part of hemoglobin in red blood cells; as it carries oxygen from the lungs to all parts of the body and facilitates oxygen use and storage in muscles. In addition, every cell in the body needs iron to produce energy [7].
Almost one-quarter of the worldwide population are affected by anemia, of which iron deficit is the primary cause [8,9]. Its deficiency is linked with impaired physical work ability, reduced mood and cognitive function, and poor pregnancy related outcomes [10,11]. An individual’s iron condition falls on a range (ranging from replete to depleted iron stores), iron deficiency and iron deficiency anemia. Therefore, individuals with iron deficiency are at increased risk of developing iron deficiency anemia [12]. In spite of advances in healthcare, iron deficiency remains a foremost public health fear in both developed and developing countries, with adolescent women being mainly susceptible. In industrialized countries, iron deficiency should be easily identified and treated, and yet it is often overlooked by medical practitioners or not recognized by women as a concern [13]. Thus, even in these countries the prevention and treatment of iron deficiency in innovative approaches are required [14].
The recommended nutrient intakes of an individual’s to meet the defined requirement vary, depending, among other factors, on the criterion used to define nutrient adequacy [15-17]. For many nutrients to scientifically support the definition of nutritional needs across age ranges, sex and physiologic states, the information base is limited. The use of nutrient balance to define requirements has been avoided whenever possible, since it is now generally recognized that balance can be reached over a wide range of nutrient intakes. However, requirement levels defined using nutrient balance have been used if no other suitable data are available. The dietary requirement for a micronutrient is defined as an intake level which meets specified criteria for adequacy, thereby minimizing risk of nutrient deficit or excess. Measures of nutrient stores or critical tissue pools may also be used to determine nutrient adequacy. Functional assays are presently the most relevant indices of subclinical conditions related to vitamin and mineral intakes. Anaemia, the defining marker of dietary iron deficiency, may also result from, among other things, deficiencies in folate, vitamin B12 or copper [18].
Individual dietary inhibitory and enhancing factors exert profound influences on iron absorption [19-21]. Polyphenol compounds are widely present in the human diet as components of fruits, vegetables, spices, pulses and cereals, and they are especially high in tea, coffee, red wine, cocoa and the different herb teas. The potent inhibitory effect of phytic acid on nonheme-iron absorption is well known [22-24]. Polyphenolic compounds such as chlorogenic acids, monomeric flavonoids, and polyphenol polymerization products widely present in coffee and tea also strongly inhibit dietary nonheme-iron absorption [25-27]. The effects of ascorbic acid (AA) in dramatically improving iron absorption have consistently been observed [28]. Heme iron found in animal foods is also an important iron source because of its high bioavailability. In addition, many studies have suggested that the enhancing effect of muscle tissue on iron absorption is due to cysteine-containing peptides [29].
The biochemistry of iron metabolism in human digestive system
Iron chemistry and physiology
Iron is found on the 26th element of the periodic table and has a molecular weight of 55.85. It has two ordinary aqueous oxidation states namely ferrous (Fe2+) and ferric (Fe3+). These states enable iron to take part in oxidation/reduction reactions that are crucial to energy metabolism by accepting or donating electrons. Oon the other hand, this property also enables free iron to catalyze oxidative reactions, ensuing in reactive and damaging free radicals. Hence, body iron required to be chemically bound to assist appropriate physiological role, transport, and storage, with minimal opportunity for free ionic iron to catalyze harmful oxidative reactions [30].
The majority of the body’s iron functions in heme protein complexes that transport oxygen as hemoglobin and myoglobin. Approximately two-thirds of the body iron is in hemoglobin, a 68,000MW structure containing four subunits of heme, a protoporphyrin ring with iron in the center, and four polypeptide chains (two chains each of α- and β-globin). For transport by hemoglobin, oxygen bonds directly to the iron atom, stabilized in a Fe2+ oxidation state surrounded by the protoporphyrin ring and histidine residues. Hemoglobin iron easily binds and releases oxygen, circulating in blood erythrocytes. Myoglobin, consisting of a single heme molecule and globin, enables oxygen transfer from erythrocytes to cellular mitochondria in muscle cytoplasm [30]. Lesser amounts of iron in the heme form function in mitrochondrial cytochromes involved with electron transfer, oxygen utilization, and the production of ATP. A small fraction of body iron functions in heme-containing hydrogen peroxidases such as catalase that protect against excessive hydrogen peroxide accumulation by catalyzing its conversion to hydrogen and oxygen [30].
In addition, iron also has functions in non-heme proteins that contain an iron–sulfur complex (a cubical structure of four iron and four sulfur atoms). It’s the principal form of iron in mitochondria that functioning in enzymes of energy metabolism such as aconitase, NADH dehydrogenase, and succinate dehydrogenase. Aconitase is sensitive to iron concentrations in mitochondria and cytosol. When iron is abundant, the aconitase enzyme assumes the full iron–sulfur cubic structure that is associated with carbohydrate metabolism. However, when iron concentrations are reduced, the protein loses aconitase activity and functions as an iron binding protein (IRP). IRPs interact with iron response elements (IREs) of the mRNA to regulate the synthesis of proteins involved with iron transport, storage, and use, in response to changes in cellular iron concentrations [30] (Figure 1).
Iron absorption and metabolism
The new born infant has a total of about 250mg in the body. The total body iron in an adult male is 3000 to 4000 mg. In contrast, the average adult woman has only 2000-3000 mg of iron in her body. This difference may be attributed to lesser iron reserves in women, lower concentration of hemoglobin and a smaller vascular volume than men. Of this approximately two-thirds are utilized as functional iron such as that in hemoglobin (60%), myoglobin (5%) and various heme and nonheme enzymes (5%). The remainder is found in storage as ferritin (20%) and hemisoderin (10%) [31].
Control of iron uptake is undoubtedly of principal importance due to the lack of a regulated means of excreting iron. Once the food is consumed and digested, dietary iron is mainly absorbed in the duodenum and proximal jejunum. Reasonably, haem iron is absorbed more efficiently than non-haem iron, apparently by endocytosis of the intact iron–protoporphyrin complex at the enterocyte brush border. After the digestion iron from all dietary sources enters a common intracellular pool from which depending on the iron status of individuals it is either stored as ferritin in the enterocyte or exported from the enterocyte via the ferroportin transporter on the basal side of the cell. Ferroportin transports ferrous iron which is immediately oxidized to Fe3+ and picked up by transferrin to be transported to cells expressing transferrin receptors (Figure 2).
Iron status, iron deficiency and iron overload
Infants, children, teenagers, and women of childbearing age are commonly affected by iron deficiency; whereas healthy adult males are seldom deficient. Deficiency is caused by several factors, usually by a combinations of increased need (rapid growth in the young population, menstruation and pregnancy in fertile women) and insufficient uptake, which in turn may depend on other factors such as a decreased caloric intake and/or a larger fraction of calories derived from food ingredients which contains less absorbed iron [32].
The earliest stage of iron deficiency is characterized by loss of storage iron (indicated by ferritin) and is called iron depletion or prelatent iron deficiency. The concentrations of serum iron and the iron-carrying serum protein transferrin are normal at this stage. When iron stores are exhausted (serum ferritin <12μg/L), serum iron decreases and serum transferrin, which is usually measured as total iron-binding capacity (TIBC) increases. If the hemoglobin concentration is still normal, this stage is called iron deficiency without anemia or latent iron deficiency. When the iron stores are completely exhausted, there is not sufficient iron for hemoglobin synthesis. This final stage is called iron deficiency anemia or manifest iron deficiency [32].
Symptoms frequently associated with iron deficiency anemia include palor, weakness, fatigue, dyspnea, palpitations, sensitivity to cold, abnormalities in the oral cavity and gastrointestinal tract, and reduced capacity for work [33]. It further appears that even mid/prelatent iron deficiency may have significant health consequences which can be attributed to decreases in essential body iron and limitations in tissue oxidative capacity [34-36].
Iron overload is associated with increases in non-protein bound iron resulting from the physiologic iron-binding capacity being overwhelmed [37]. Disadvantages with overload are for example increased risk for bacterial infection and cardiomyopathy. Overload can result from inborn errors in metabolism leading to hyper-absorption of iron or inadequate synthesis of the iron-binding proteins. Overload can also result from excessive absorption of dietary iron due to various causes including chronic ingestion of greater than adequate amounts of dietary iron, especially heme iron. These observations concerning of iron overload have raised the question as to whether or not general fortification of food with inorganic iron is beneficial [37].
Dietary and non-dietary factors affecting iron absorption
Dietary factors contribute a significant role in the development of iron deficiency and then iron deficiency anemia. Iron absorption by the gut enterocytes controls iron balance but there is no route of controlled iron excretion. This means that iron absorption is regulated by dietary and systemic factors. Dietary iron is largely non-heme iron with about 5%–10% in the form of heme iron in diets containing meat. Even though heme iron constitutes a smaller part of dietary iron, it is highly bioavailable and 20%–30% of heme iron is absorbed. Contrary, the absorption of non-heme iron is much more variable and significantly affected by other components of the diet; with 1%–10% of non-heme iron absorbed [38].
Moreover, iron in the environment and the diet is primarily ferric iron (Fe3+) which is insoluble and so not bioavailable. Thus, before it can be absorbed the non-heme iron has to be reduced from ferric (Fe3+) to ferrous (Fe2+) iron by dietary reducing agents, such as ascorbic acid or by endogenous ferri-reductases, such as duodenal cytochrome B (dcytB) [39]. Ferrous iron is transported across the apical membrane of the duodenum by the divalent metal transporter 1 (DMT1), which is localized on the brush border membrane close to dcytB. As Wang & Pantopoulos, 2011 reported, the uptake of ferrous ions by dcytB is driven by proton co-transport, so an acidic duodenal pH facilitates iron uptake, and is competitively inhibited by other divalent cations.
Ascorbic acid is one of the most effective enhancer of non-heme iron absorption. Other dietary factors such as citric acid and other organic acids, alcohol and carotenes similarly enhance non-heme iron absorption [38]. Furthermore, animal based proteins such as meat, fish, and poultry, enhance iron absorption but the bioactive component of the “meat factor” has yet to be identified. Meat also promotes non-heme iron absorption by activating gastric acid production. Conversely, absorption of non-heme iron is inhibited by phytic acid (inositol hexaphosphate and inositol pentaphosphate) in grains and cereals and by polyphenols in some vegetables, coffee, tea, and wine. These inhibitors bonded to non-heme iron so it is not available for uptake. Dietary factors influencing iron absorption are outlined in Table 1.
Dietary factors inhibiting iron absorption
Calcium: Calcium does inhibit both non-heme iron and heme iron absorption. Calcium inhibits the absorption of both heme and nonheme iron in a comparable way and thus, it is likely that this inhibition by calcium occurs after the heme iron is freed from the porphyrin ring [40]. Calcium has been shown to inhibit iron absorption in both rats [41] and man [42-45], reported that giving 165 mg Ca as milk, cheese or calcium chloride reduced iron absorption by 50-60% in single-meal measurements with maximum inhibition of approximately 300 mg calcium in the meal. Further increasing the amount of dairy products above a basal level of 300 mg appears to have no further inhibiting effect on iron absorption (Galan et al. 1991). However, the duration of the inhibitory effect of calcium on iron absorption has been shown to be less than two hours [46]. On the other hand, ingestion of 1000 mg Ca as the carbonate daily with meals over a twelve-week period did not appear to be harmful to their iron status [47].
Phytate: During digestion, the phytate molecule can be negatively charged, indicating a potential for binding positively charged metal ions like iron [48]. The negative consequence of phytate in bran on iron absorption was first demonstrated by Sharpe et al. (1950)[49], using white bread and brown bran bread. This effect was earlier supposed to be because of its high content of phytate which has been demonstrated in a number of more recent studies [23,50]. Brune et al. (1989)[51] investigated the likelihood to long-term consumption of high bran containing phytate diet could induce changes in the intestine which would bring about an adaptation to the inhibitory effects of phytate on the intestinal iron absorption. They examined vegetarians and non-vegetarians and found that no adaptation of intestinal brush border to a high phytate intake and concluded that the satisfactory iron stores in the vegetarian group were due to a high consumption of organic acids like ascorbic acid.
Several methods of preparations of cereal grains including soaking, germination and fermentation have been shown to completely reduce the phytate content of cereals and vegetables under optimal conditions [52] and could thereby eliminate their effects on iron absorption. In addition, negative effects of phytate and fiber on iron absorption have been demonstrated in the rat [53], found a reduction in iron absorption when high fiber breads were fed to rats. However, the magnitude of this inhibition was unrelated to the amount of phytate phosphorus or dietary fiber present in the diet.
In contrary, results from experiments by [54], indicated higher absorption from FeSO4 than from the endogenous Fe present in bread, both expressed as mg Fe absorbed and fractional Fe absorption. Using balance and single meal radioisotope measures, she found no differences in Fe absorption among three different breads with fiber contents of 16.1, 38.1 and 72.4 g/kg but with the same phytate concentration (6.3-6.4 g/kg) [55], used the exact same bread in balance measures in humans during 3 x 24 days in 6 subjects and did not find any influence of fiber concentration on iron balances. This indicates indirectly that the inhibitory effect of fiber on iron absorption is probably due to the phytate in the fiber fraction and further supports the study of Morris and Ellis (1980)[56], who found that iron absorption in rats was higher from dephytinized bran.
Phenolic Compounds: During digestion, the phenolic compounds from the food or beverage released and can form complex with Fe in the intestinal lumen making it unavailable for absorption. Nearly all beverages reduced iron absorption depending on the amount of total polyphenols, with the inhibition of black tea the greatest at 79–94%. Few studies revealed that an amounts of only 20 mg polyphenols from black tea per meal reduced iron absorption by as much as 66%, possibly because of the higher content of galloyl esters in black tea and probably because the simple bread meal was devoid of any enhancers of iron absorption to counteract the polyphenols (Prashanth et al., 2008). Similarly, the consumption of black tea and coffee has been shown to strongly inhibit iron absorption from composite meals [16,25,26], with coffee having about half the inhibitory effect of tea.
Mathematical Model
The basic core for mathematical model was taken from the coupled Van der Pol model of the heart electrical activity, given in the paper [4]. Trying to simplify the solutions of the NSE and KdV there were used nonlinear second order differential equations with odd non linearity for NSE and even non linearity for KdV equation.
The coupled Van der Pol equations by agreeing coefficients were additively linked to the equations substituting NSE and KdV, forming a new model looking in the following way:
Where - is the magnitude proportional to the dynamic electric potential of the whole myocardium, - is the magnitude proportional to the dynamic electric potential of the myocyte locality on the surface of the myocardium, - is the magnitude proportional blood pressure in the small arterial network, - is the magnitude proportional blood pressure in the magisterial arterial network, - agreeing coefficients, ,constant coefficients, - is the magnitude proportional to the time lag which requires the propagation of the electric impulse in the myocardium, - is the magnitude proportional to the time lag which requires the propagation of the electric impulse in the myocyte locality, - is the magnitude proportional to the square of the myocardium, - is the magnitude proportional to the square of the myocyte locality, - is the magnitude proportional to the time of the myocardium contraction, - is the magnitude proportional to the period of oscillations in the myocyte locality, - are the beating frequencies equal to 1, - is the perturbation function of the resonant external medium effect on the heart at the frequency of about 1 Hz, - is the perturbation function of the resonant external medium effect on the heart at the frequency of about 20 Hz. , - are the constants.
The study of the model
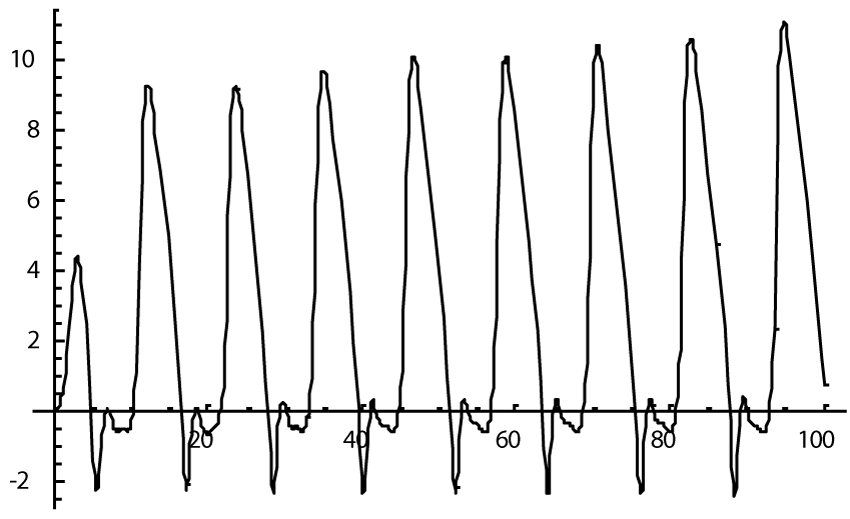
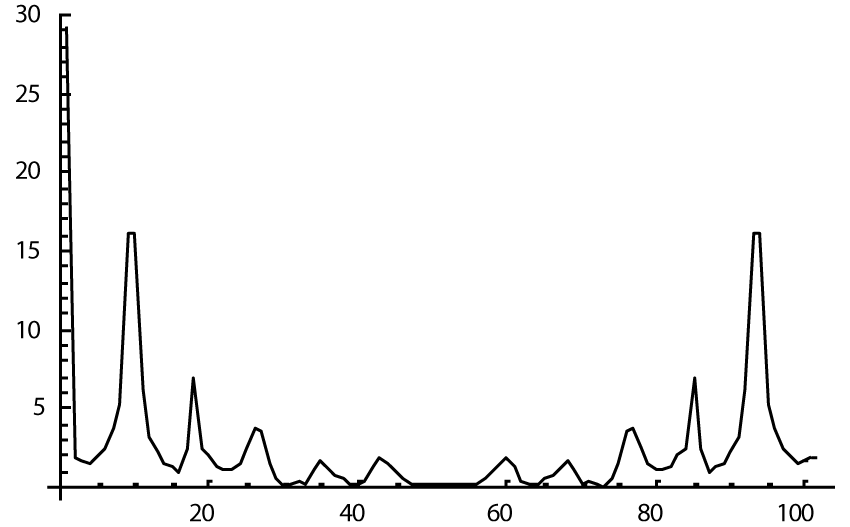
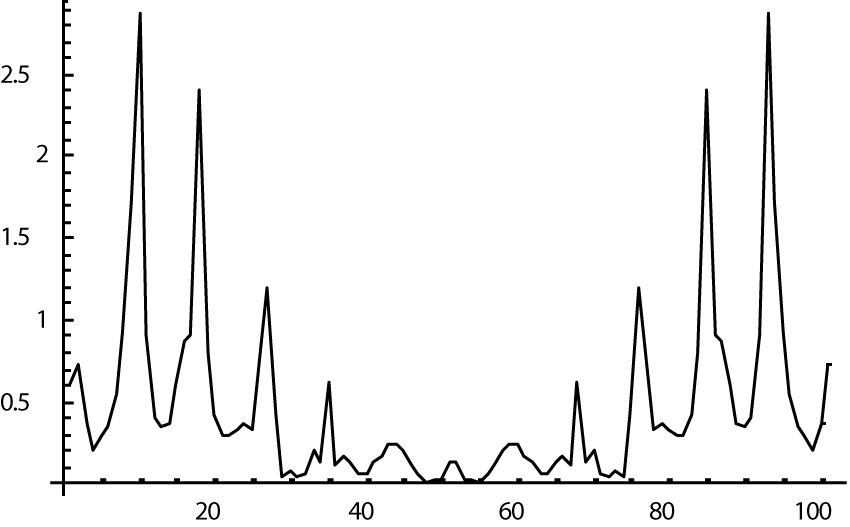
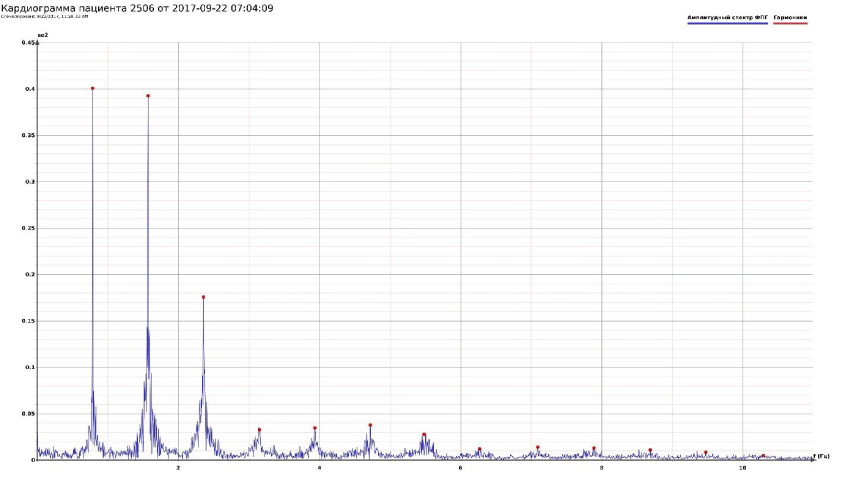
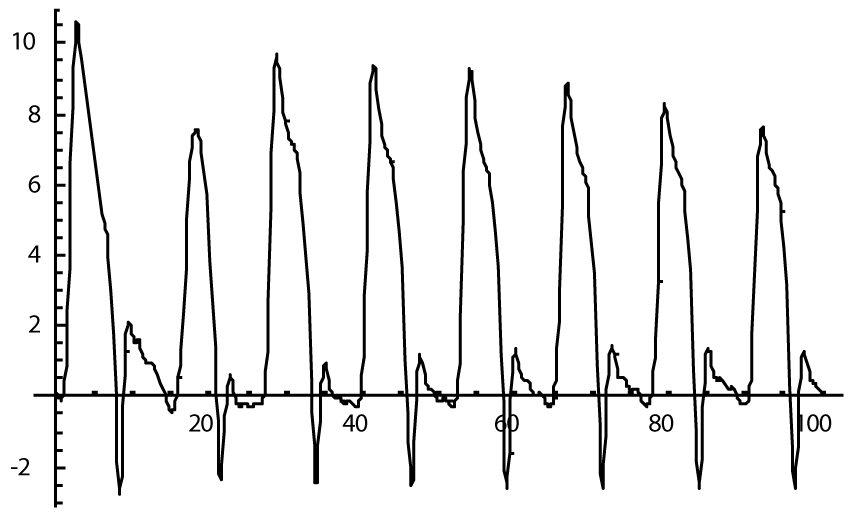
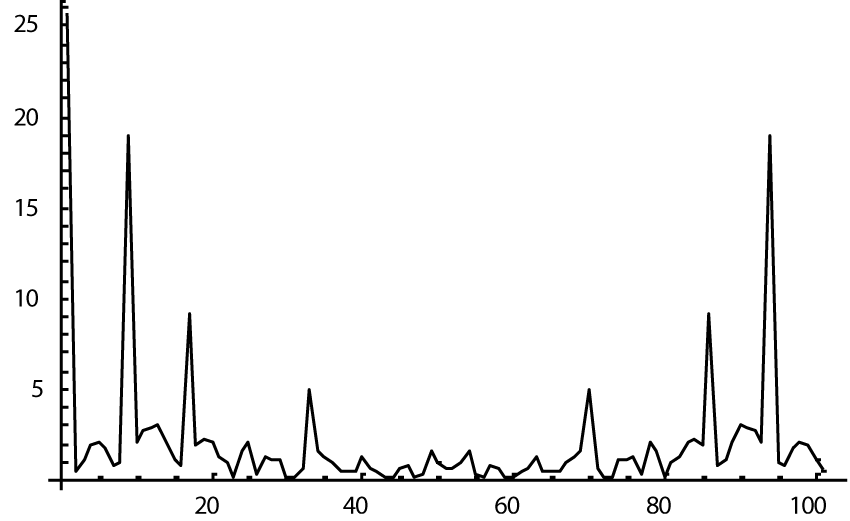
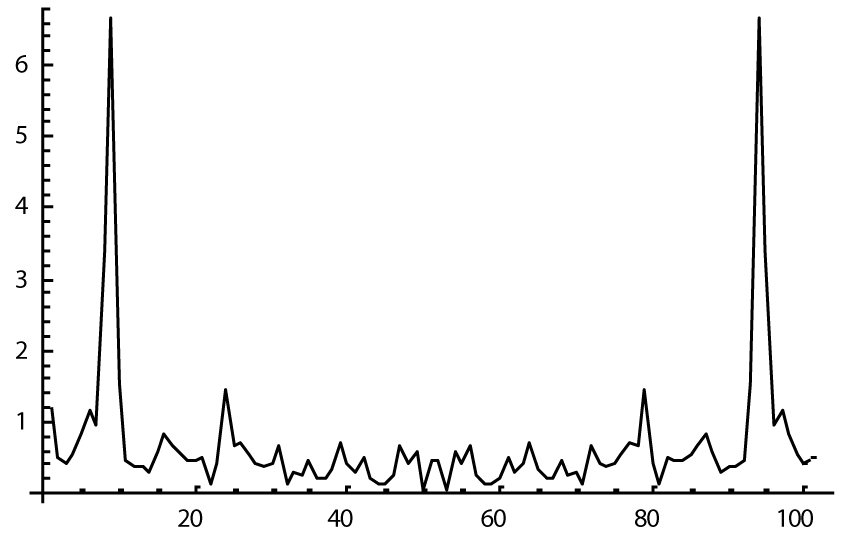
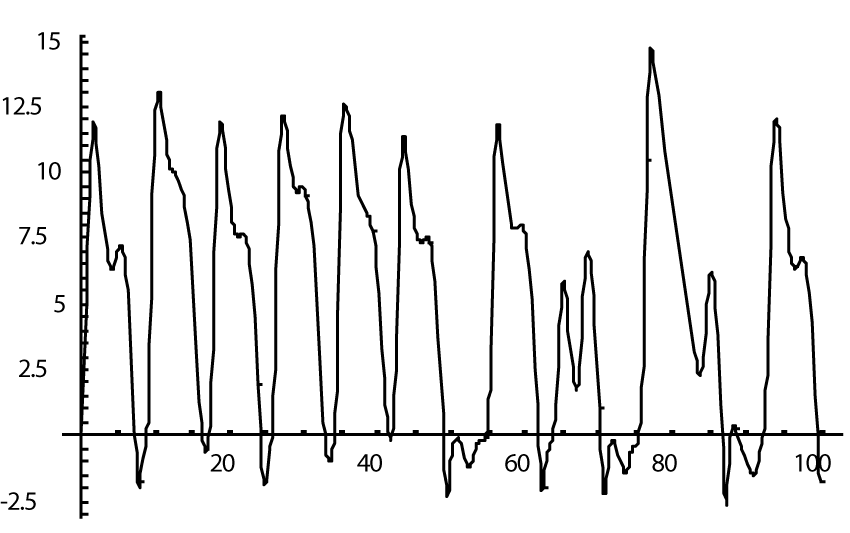
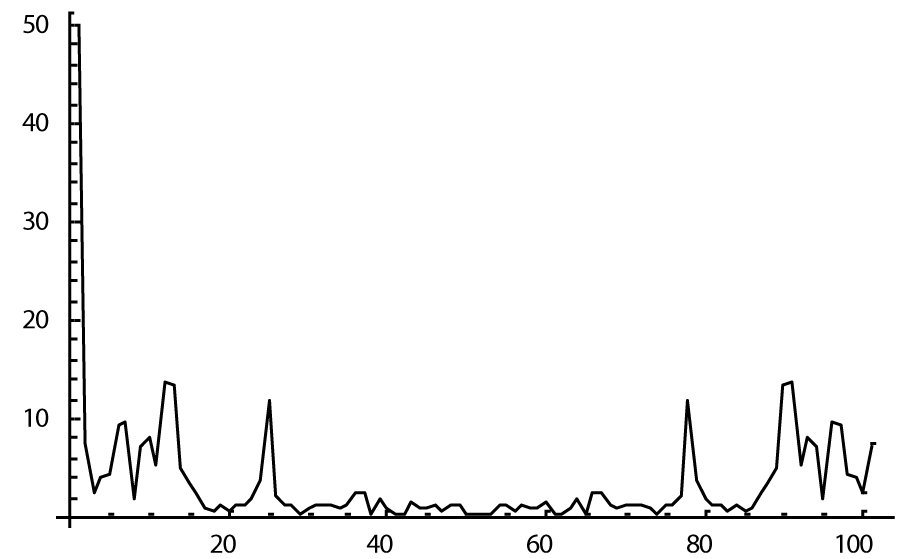
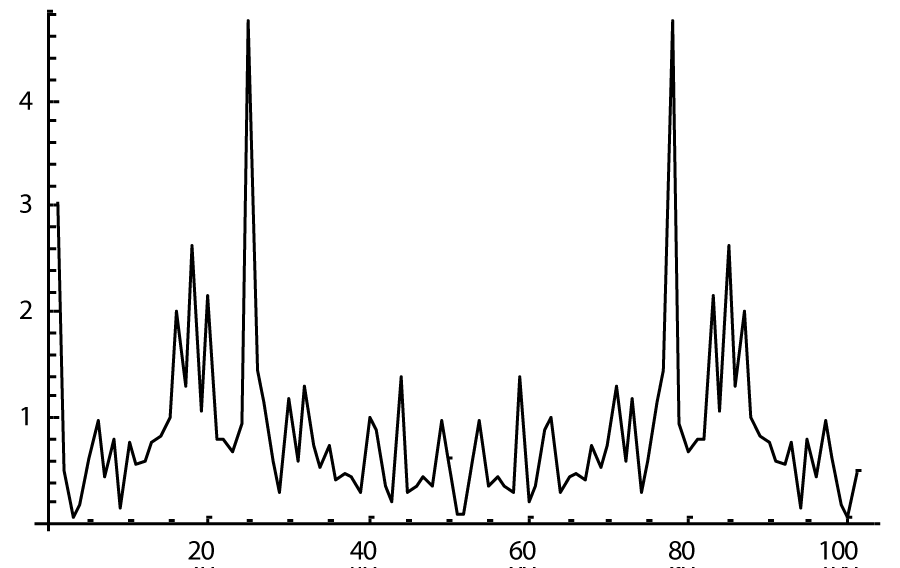
The computer study of the developed model allowed getting the model solutions corresponding to the healthy state of the heart as well as magisterial and small arteries networks coupled functioning. Figures 1-3 show model ECGs and Pulse Wave Fourier images correspondingly.
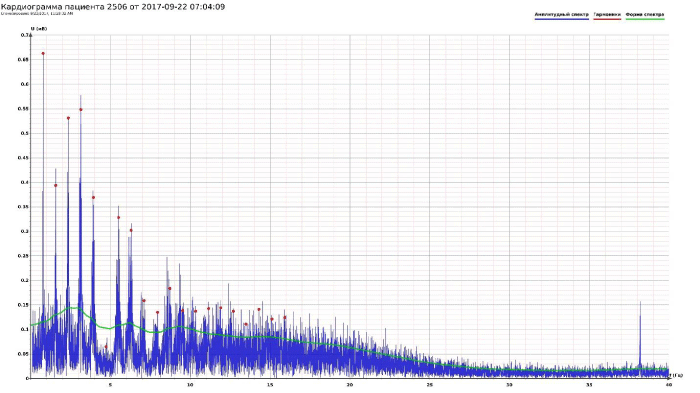
The model Fourier images were compared with the ECG and Pulse Wave Fourier data of healthy patients. As it can be seen from the graphs (Figures 4,5), the model Fourier pictures of a healthy patient correspond to the mathematical model solutions.
Next step of modeling was to decrease the myocardial surface by 50% as a model of myocardial infarct. Previous model [4] showed close to fibrillation pictures whereas modeling such situation within a new model (1) demonstrated only a small deterioration of the ECG picture (Figure 6), and its Fourier image (Figure 7), as well as the images of the magisterial and small arteries networks dynamics (Figure 8).
Modeling of fibrillation within a new model proved an ability of the coupled system of the heart and magisterial and small arteries networks to withstand the cardiovascular problems happened due to different reasons. In particular, creating model fibrillation in a new model requires five times larger magnitude of the amplitude of external influence to compare with the magnitude of the amplitude in the previous modeling the state of heart fibrillation without the influence of arterial networks [4]. The arterial networks stabilize the state of heart functioning (Figures 9-11).
Discussion
The new mathematical model of the coupled system of the heart and magisterial and small arteries networks interacting demonstrated the unified stable working of the system and its increased ability to resist the development of cardiovascular problems such as myocardial infarction. The modeling of blood pressure dynamics in small arteries shows its ability to maintain the necessary blood pressure there as well as to buffer its sharp growth in cases of emotional stress. At the same time, the self-parametric excitation in the equations modeling the dynamics of blood pressure in small arteries points at their principal possibility to blow up healthy small arteries in some anomalous cases causing a brain hemorrhage or a heart attack.
- Novopashin MA, Shmid AV, Berezin AA (2017) Fermi-Pasta-Ulam auto recurrence in the description of the electrical activity of the heart. J. Medical Hypothesis 101: 12-16. Link: https://goo.gl/scAs6T
- Yuen HC, Lake BM (1982) Non-linear dynamics of deep-water gravity waves. Advances in applied mechanics 22: 67-229. Link: https://goo.gl/SjfMmD
- Zabusky N, Kruskal M (1965) Interaction of solitons in a collision less plasma and recurrence of initial states. Physical Review Letters 15: 6-9. Link: https://goo.gl/SYZAF7
- Novopashin MA, Shmid AV, Berezin AA (2017) Forrester’s Concept in Modeling Heart Dynamics. IOSR Journal of Computer Engineering Link: https://goo.gl/A12B9N
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
