Advances in Toxicology and Toxic Effects
Codeine induced hematological, hepatic alterations, lung and brain damage in mice
Bernard Omokheshi Adele*, Adeyinka Joseph Alonge and Elsie Olufunke Adewoye
Cite this as
Adele BO, Alonge AJ, Adewoye EO (2022) Codeine induced hematological, hepatic alterations, lung and brain damage in mice. Adv Toxicol Toxic Effects 6(1): 001-007. DOI: 10.17352/atte.000011Copyright
© 2022 Adele BO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Codeine, an opiate derivate, which induces pleasure and euphoria in users, is contained in many OTC cough syrups as dextromethorphan. In 2011, its abuse has been reported in Nigeria from consumption of codeine-based cough syrup such as Benylin containing codeine syrup (BCS). Thereafter, the neurobehavioural alteration was reported with BCS in mice. 45 Swiss male mice (20 g -25 g) were grouped into control, low dose-(10.95 ml/kg BCS) and High dose-(21.90 ml/kg of BCS). BCS was given orally above the therapeutic dose for 4weeks. Blood samples were collected after 7 and 28days under mild ether anesthesia into plain and heparinized bottles to assess hematological indices, serum creatinine level, and activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Thereafter, the brain, lung, and liver were excised and processed for brain protein level and histopathological observation. Data were analyzed using Two-way ANOVA at P < 0.05. At both doses, BCS reduced hemoglobin concentration (10.56; 21.69%), lymphocyte count (10.54; 29.22%) and brain protein level (4.86±0.81; 4.86 ± 0.80 vs 9.20 ± 0.61 g/l) while white blood cell count (20.47; 46.08%), serum creatinine level (5.36; 18.75%), AST (26.31; 32.77%) and ALT (22.90; 36.70%) activities were increased compared to control. Histology shows marked necrosis and chronic infiltration by inflammatory cells in the brain, liver, and lung. Acute and chronic treatment of mice with Benylin with codeine resulted in significant alterations in blood and vital body organs such as kidney, liver, lung, and brain in a dose-dependent manner.
Introduction
Drug use and its abuse is an age-long practice that has been common to mankind for centuries. Opiods constitute one of such groups of drugs that have been used for years by men in the management of acute and chronic pains [1,2]. Opioids interact with their receptors in the brain and spinal cord to decrease pain perception and stimulate the intense sensation of euphoria or well-being. These feelings of euphoria and sedation in users became increasingly sought after and lead to the abuse of opioids [3,4]. Codeine is an opiate derivate, a prodrug of morphine, and a narcotic with higher numbers of reported drug misuse-related deaths in the USA as of 1991 [5]. Wanner, et al. [1] reported that codeine abuse can result in addiction and as well increase the risk of relapse in treated users addicted to other drugs.
Codeine ((5α, 6α)-7, 8-didehydro-4, 5-epoxy-3-methoxy-17-methylmorphinan-6-ol) is found in most over-the-counter (OTC) cough syrups as codeine or its analog - Dextromethorphan (3-methoxy 17 –methylmorphian) [6] Its abuse is facilitated by the internet and access to this syrup in pharmaceutical outlets without prescription. In Nigeria, it is abused from consumption of Benylin with codeine cough syrup which contains codeine phosphate, levomenthol (nasal decongestant), and diphenhydramine (an antihistamine) indicated for the relief of persistent, dry, and irritating cough [7,8]. In contrast to its indicated use and prescription guidelines, Benylin with codeine is consumed by South-Western and Northern Nigerian youths (especially between 17-45 years) for recreational purposes due to the addictive nature of the constituent opiate [7]. Consumption of this syrup above the maximum daily dose of 40 ml for adults is abuse. According to UNDOC, World Drug report in 2018 [9], cough syrups (containing codeine or its analog) were found to be the third most abused drug leading to the subsequent ban of syrup in Nigeria.
The indiscriminate consumption of Benylin-codeine cough syrup either repeatedly or once was reported in experimental settings to impair motor and mental activities in mice [8] while the psychotoxic and reproductive effects of dextromethorphan (codeine analog in other OTC cough syrups) have also been reported [10,11]. The motor and cognitive function of the brain is dependent on the interplay between different brain protein components. These proteins are brain resident enzymes, nerve cells, immune cells, membrane receptors, neurotransmitters, etc. that function together as the components of the underlying mechanisms. The reported impairments in brain functions [8,10] could be associated with brain protein derangement. More so, the liver remains the primary target organ constantly challenged with the metabolism of food nutrients, drugs, and xenobiotics, until the kidney was included recently [12]. This metabolic function is altered in the event of overwhelming plasma concentrations of the drugs/xenobiotics and/or turn-over of toxic metabolites [13]. Codeine is hepatically converted to morphine (codeine active metabolites) and morphine-6-glucuronide [14]. The later metabolite mediates respiratory depression and nausea and is also linked with narcosis in patients when renal clearance fails [15]. Since plasma clearance of codeine and its metabolites are important to prevent toxicities, it is therefore unlikely that excessive consumption of codeine from cough syrups will not overwhelm hepatic and renal functions. This study, therefore, examined the acute and chronic effects of repeated consumption of Benylin-containing codeine syrup on blood parameters, liver, and renal functions, brain total protein, and lung and brain microscopy in male mice.
Materials and methods
Animals and experimental design
Forty-five Swiss male mice (20 g- 25 g) were obtained, acclimatized to laboratory conditions, and grouped into three (3) of 15 animals each: 1(control) received 10ml/kg distilled water while 2 (low dose) and 3 (High dose) received an oral dose of 10.95 ml/kg/day and 21.90 ml/kg/day of Benylin with codeine [8] respectively for four weeks. All animals were maintained on standard chow and water ad libitum. All experiments followed the guidelines of the Animal Care and Use Research Ethics Committee (ACUREC), University of Ibadan, Nigeria, and that of the Guide for the Care and Use of Laboratory Animals (NRC,1996), published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA. Doses used were extrapolated based on the maximum therapeutic dose of 40 ml/day for adults, LD50 for codeine in rats (427 mg/kg), and also followed that of Tijani, et al. [8].
Biochemical assay
A blood sample (3mls) was collected through retro-orbital puncture under mild ether anesthesia into plain and heparinized bottles after 7 and 28 days of treatment. Plain blood samples were centrifuged after 1 hour at 3500rpm for 10 minutes to obtain serum while heparinized blood was immediately subjected to hematological study using a hematology auto-analyzer (Haematology auto analyzer Sysmex KX-21N). Hematological parameters analyzed were packed cell volume (PCV), red blood cell (RBC) count, hemoglobin concentration, platelet, white blood cell (WBC), neutrophils, and lymphocyte count. Neutron-lymphocyte ratio (NLR) and Platelet-Lymphocyte ratio were calculated from neutrophils, lymphocyte, and platelet values. Serum creatinine, alanine aminotransferase (ALT) activity, and aspartate transaminase (AST) activity were assessed using assay kits (Randox assay kits).
Organ collection and processing
After blood collection, animals were sacrificed and the skulls were carefully opened up to excise the brain, rinsed in ice-cold potassium chloride (pH 7.4), blotted out, weighed, and homogenized in 5X ice-cold phosphate buffer saline (0.1M, pH 7.4). The tissue homogenate was centrifuged at 13000 rpm (4 °C) for 10 minutes to obtain supernatant that was used for total protein determination.
Liver, lung and brain microscopy
Liver, lung, and brain tissues were fixed in 10% formalin for 24 hours, dehydrated using 70 -100 % ethanol, and embedded using paraffin. Thereafter, the tissues were sectioned, mounted on slides, cleared of paraffin wax, rehydrated using a decreasing concentration of ethanol (100 – 70 %), and stained using Hematoxylin & Eosin.
Statistical analysis
Data were expressed as Mean ± SEM, analyzed using one-Way ANOVA and Newman Keuls’ Posthoc test for statistical significance at p<0.05. Data were analyzed using GraphPad Prism, version 5.0 (GraphPad Software Inc., USA).
Results
Repeated administration of a high dose of Benylin with codeine significantly (P < 0.05) increased WBC count (6150 ± 204.90 vs 4210 ± 151.20 x 10mm-3), but significantly reduced hemoglobin concentration (13.72 ± 1.03 vs 17.52 ± 0.61 g/dL) and lymphocyte count (47.00 ± 4.44 vs 66.40 ± 1.03%) after 28 days compared to normal untreated. Red blood cells (RBC), neutrophils, and platelet were also increased at both doses. However, these increases were not significant (Table 1). There was a significant increase in NLR value on days 7 and 28 at both doses while low-dose treatment did not show a significant increase in PLR value on day 28 only (Table 1).
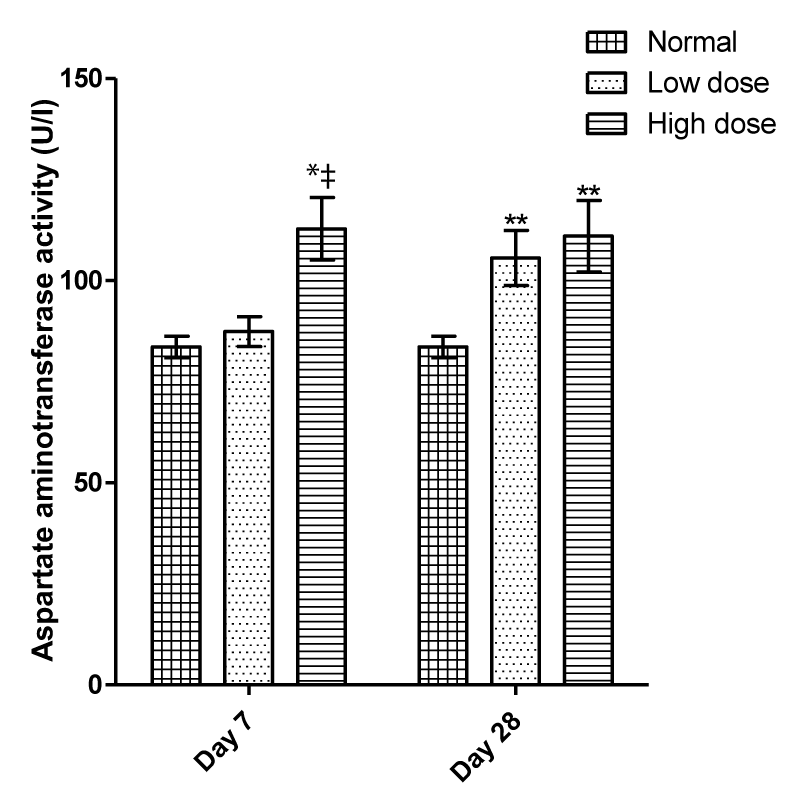
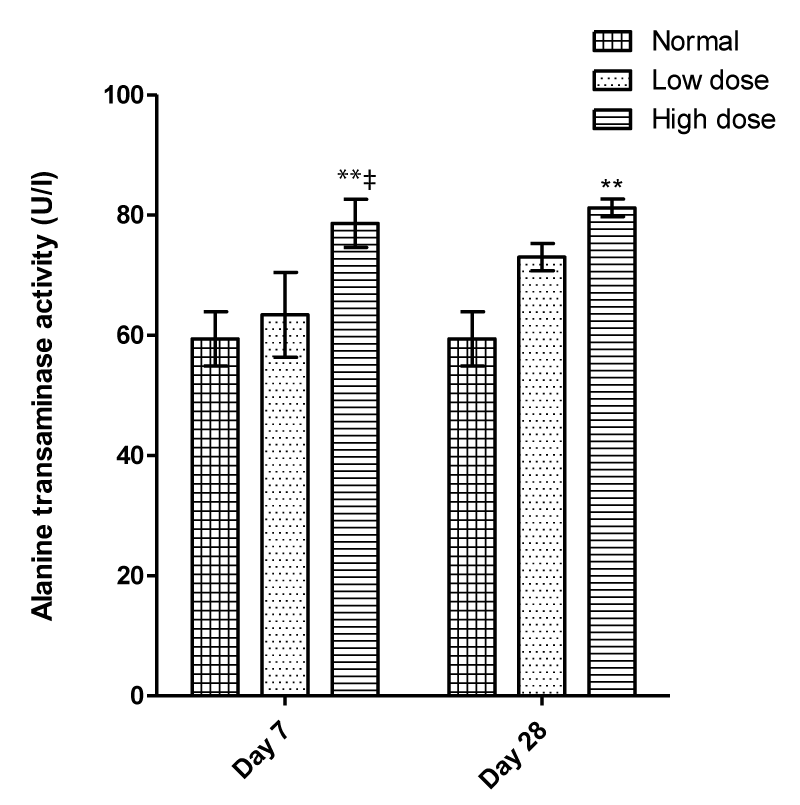
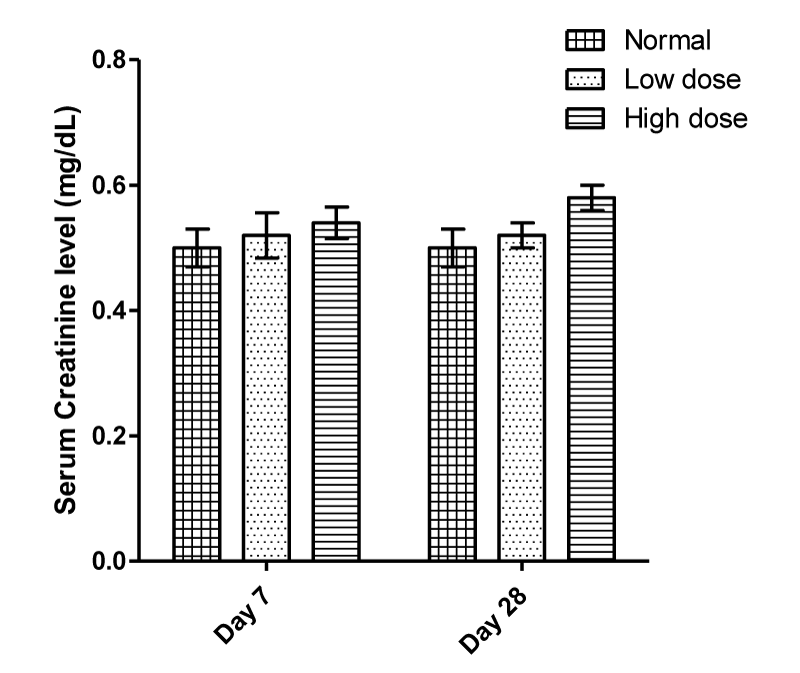
After 28 days of treatment, serum activities of AST (105.60 ± 6.83; 111.00 ± 8.86 vs 83.60 ± 2.69 U/l) and ALT (73.00 ± 2.24; 81.20 ± 1.46 vs 59.40 ± 4.53 U/l) were significantly (P < 0.05) increased at both doses compared to normal untreated (Figures 1,2). However, no significant change was observed in the value of serum creatinine level in all treated groups compared to the normal group (Figure 2). After 7 days, a significant increase in the hepatic enzymes, AST (112.80 ± 7.74 vs 83.60 ± 2.69 U/l and ALT (78.60 ± 4.01 vs 59.40 ± 4.53 U/l) were observed in high dose only. Compared to the low dose on day 7, AST and ALT activities increased significantly (Figures 1 and 2).
Creatinine level was not affected significantly, however, on day 28, both doses caused a 5.36 and 18.75 % increase respectively in creatinine level compared to control (Figure 3). These increases were not statistically significant.
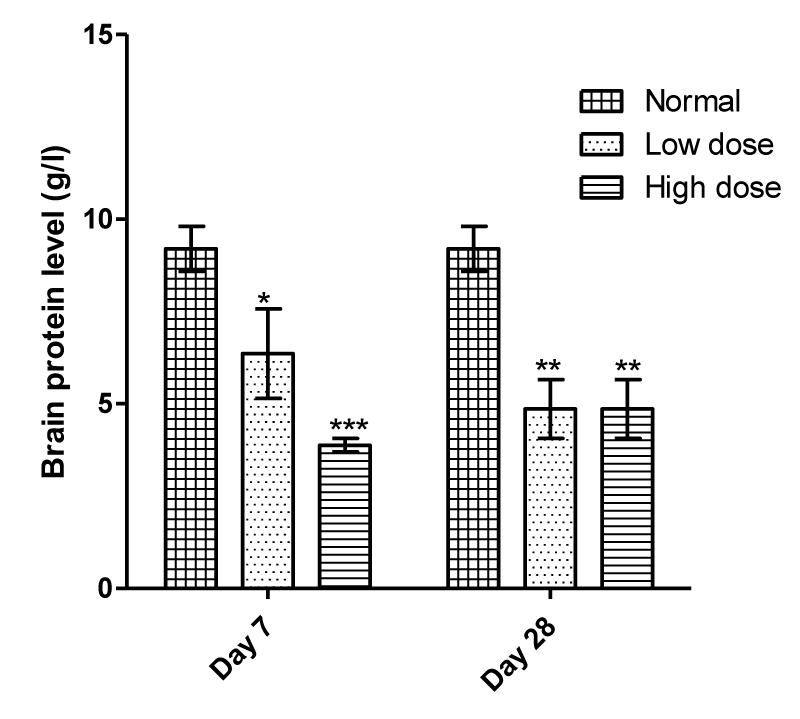
There was a significant (P < 0.05) decrease in brain total protein in both groups treated with Benylin with codeine compared to normal untreated groups (Figure 4). Compared to Group 1, the decrease recorded in the brain total protein for both doses of Benylin was 30.87 and 57.83% respectively after 7 days. But after 28 days, both doses caused a 47.17% decrease.
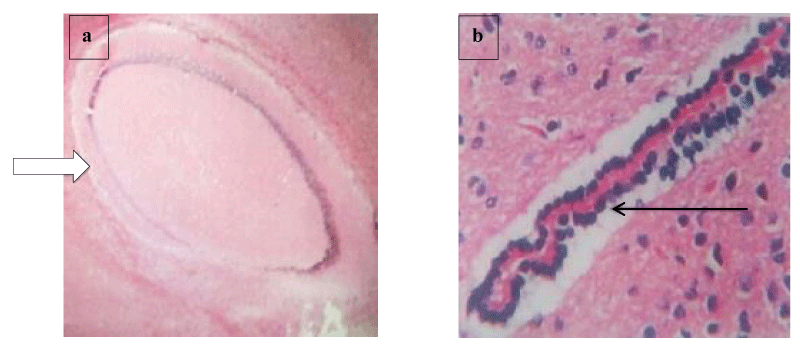
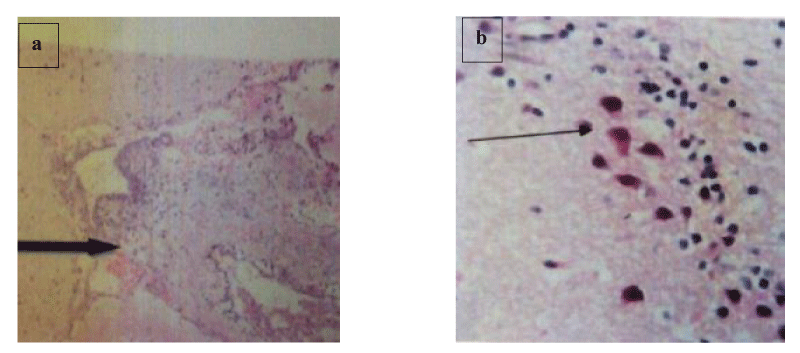
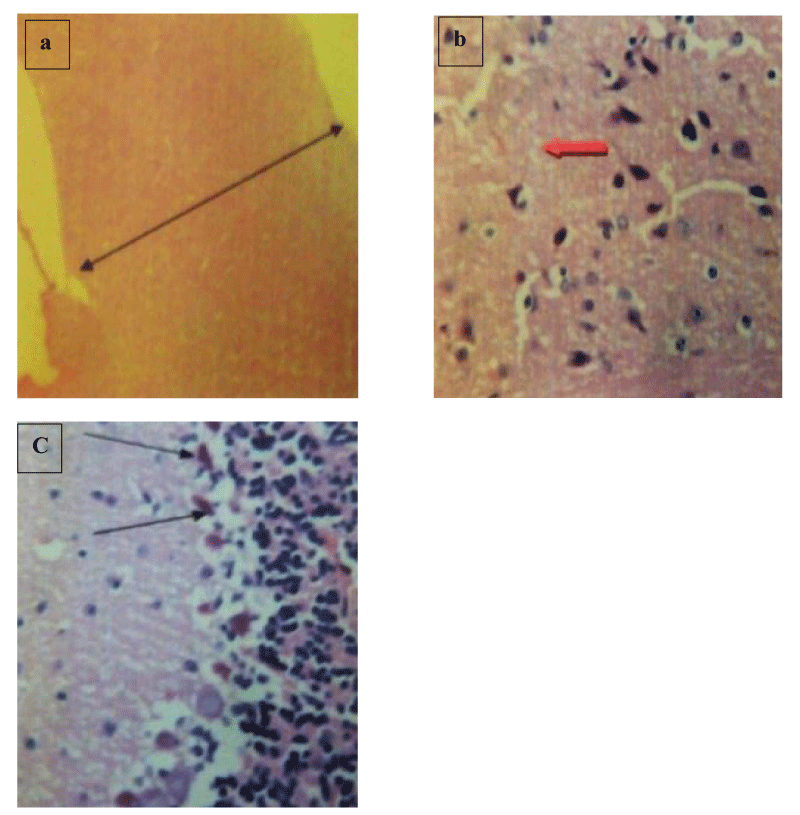
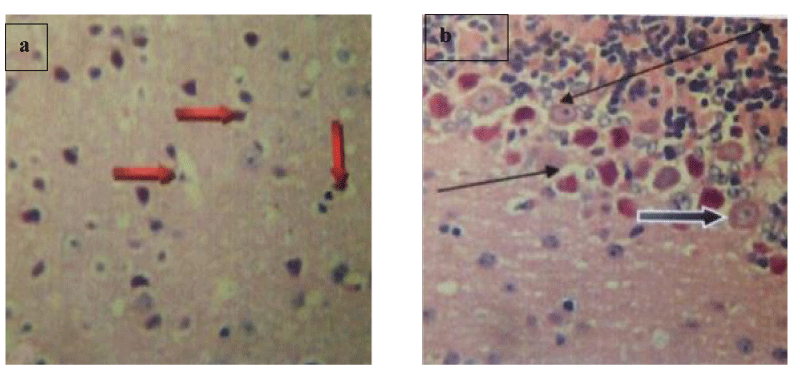
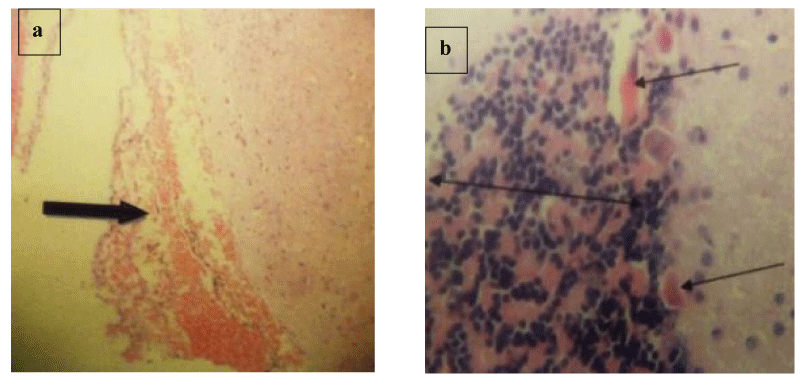
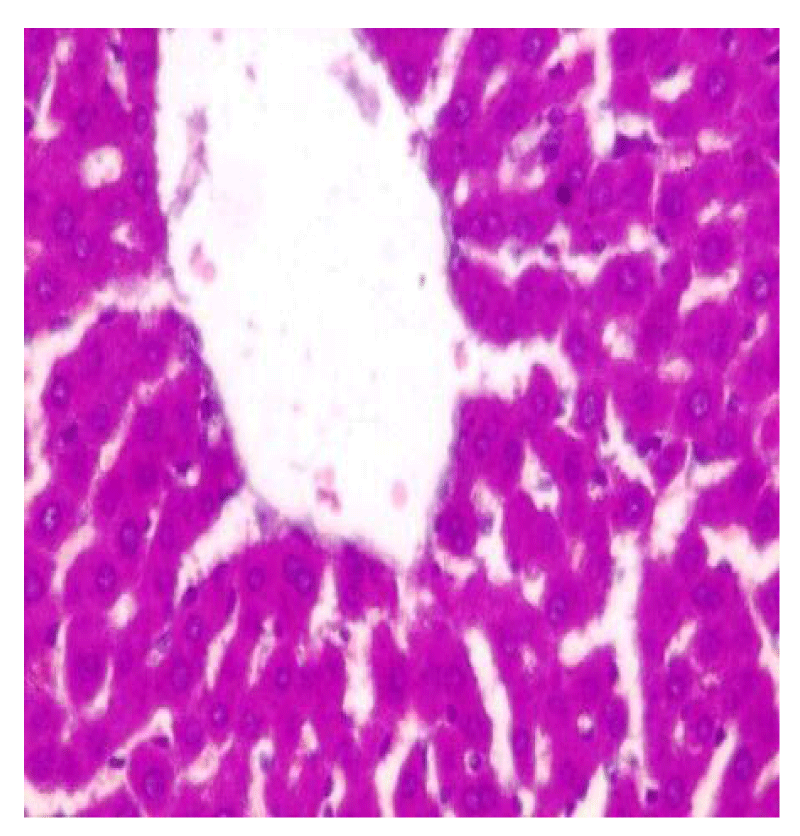
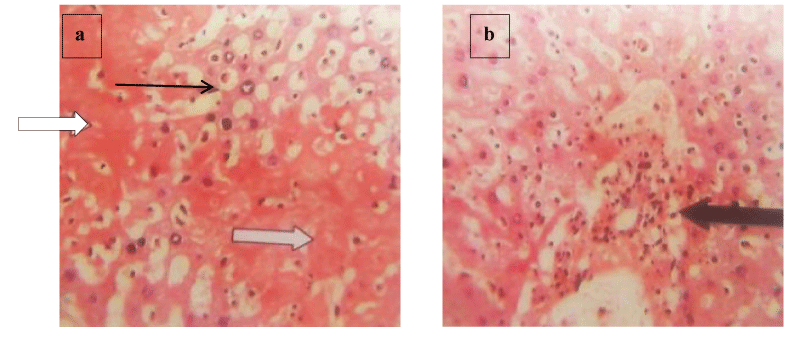
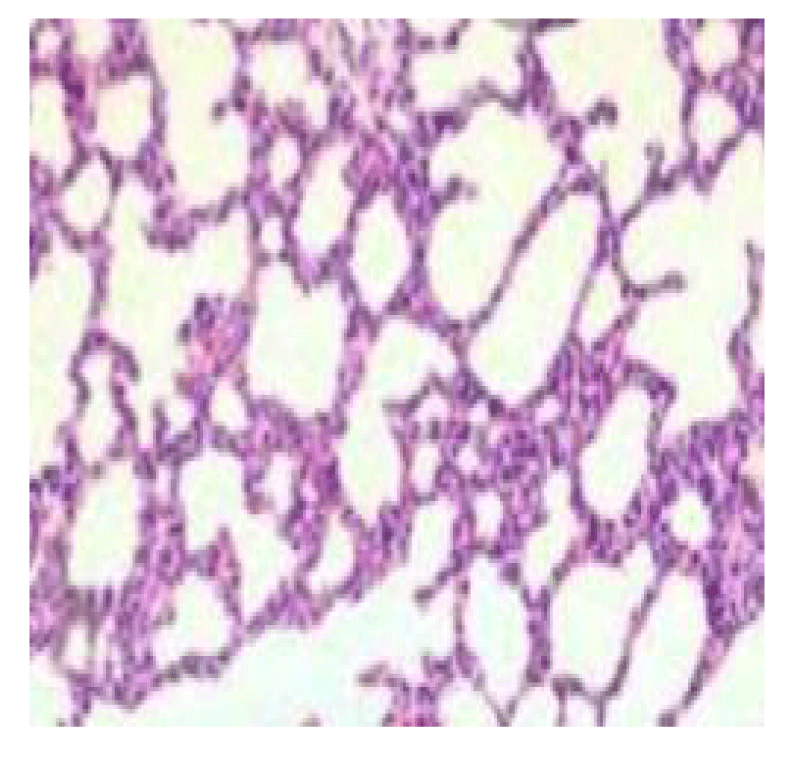
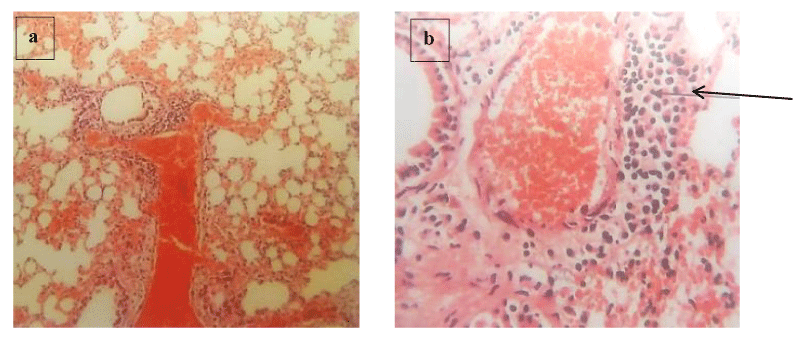
Brain histology shows necrosis of the cerebral cortex, fibrotic stroma with mild infiltrations by inflammatory cells, and shrunk and hyalinized Purkinje cells (Plates 1-5). Lung and liver microscopy showed marked necrosis and chronic infiltration by inflammatory cells (Plates 6-9).
Discussion
Drug/xenobiotic metabolism is reported to be hepatic and renal [12], and as such highly susceptible to the insults of these chemical insults [13]. Both organs use resident Nacetyltransferases, sulfotransferases, glutathione transferases, and CYP450 enzymes to metabolize drugs [12]. The liver converts a quarter of all prescribed drugs while the kidney also contributes to glucuronidation of chemical substances such as morphine, paracetamol, etc [12]. Codeine was primarily designed for the relief of pain and to enhance socio-economic life. It is a potent central analgesic, antinociceptive, antitussive, and antiperistaltic agent in the treatment of pain and cough (Bhandari, et al., 2011). Codeine is converted to its metabolites by hepatic CYP2D6 enzyme to provide its analgesic effects. An increased presence of codeine metabolites either from rapid and complete metabolism parent compounds can result in morphine overdose (Crews, et al. 2021). Hence, challenging the liver constantly with Benylin-codeine syrup from overdose or abuse can also result in morphine overdose/toxicity and may overburden renal clearance.
The liver and kidneys are involved in blood formation, immunity, and constant supply of other body organs with glucose for proper function [16,17]. Toxicity in these organs could reflect organ failure, derangement in blood, immune failure, renal insufficiency, and other complications. This study showed increased RBC count (polycythemia) accompanied by reduced hemoglobin concentration, increased WBC, and platelet count. This may be due to observed codeine-induced liver damage (Plate 6) and injury to alveolar sacs and bronchioles in the lung (Plate 7) which might have resulted in alterations in iron storage, respiratory depression, and hypoxia. Hypoxic condition stimulates erythropoietin production from the kidneys to increase RBC production while decreased iron availability alters hemoglobin synthesis and consequently reduced hemoglobin level [4,18].
An increase in WBC and a decrease in lymphocyte count with Benylin-containing codeine treatment suggest susceptibility to infection and suppression of cellular immunity [19]. Peterson, et al. [20] and Weber, et al. [21] reported that acute and chronic opioid administration suppressed antibody and cellular immune responses, natural killer cell activity, cytokine expression, and phagocytotic activity. Opioids were also implicated in the increased incidence of infections in heroin addicts and as a co-factor in the pathogenesis of the human Immuno-deficiency virus [22,23]. The result from this study agrees with the earlier report of Weber, et al. [21] who reported suppressed cellular immunity and somatic alterations in the human heroin animal model. Depressed regulatory functions of lymphocytes as a result of the reduced count will adversely affect the immune defense thereby increasing the risk of coming down with an infection [19].
WBC count and other indicators of inflammation generally increased in inflammatory disorders with platelet taking a central position in the progression. Platelets are also a non-specific first-line marker of inflammation; modulating the recruitment of neutrophils and macrophages by releasing chemokines and cytokines to sustain inflammatory response [24]. As recorded in this study, the elevated platelet count is indicative of a systemic inflammatory response [25]. Platelet-mediated inflammation has been reported to contribute to cancer progression with platelet-derived growth factors contributing to oncogenic transformation and tumorigenesis [26]. The risk models for predicting survival and/or progression of disorders include NLR and PLR values based on the role of WBCs and platelets in the initiation, progression, and complications of cardiovascular-related diseases [27] were reported to increase in this study. Higher NLR and PLR detect inflammatory activation underlying some disorders such as cardiovascular disorders, cancers, etc. [19,28], thereby indicating a possible predisposition to cardiovascular disorder (CVD) and other related complications. NLR and PLR values can also reflect balance in immune functions and predict immune and inflammatory diseases (Gao et al. 2019), and inflammation-related tumor growth (An et al. 2019). It is therefore very much unlikely that Benylin-codeine abuse will not result in cardiovascular disorders and others in later life.
According to Lee [13], continuous exposure of hepatocytes to toxic chemical substances for metabolism increases the risk of organ failure. Single and repeated consumption of codeine has been reported to induce motor and cognitive impairment [8], impair reproductive functions [11], and the possibility of altering hepatic and renal functions. Drugs account for a higher percentage of liver injury [29]. In conjunction with other diagnostic tools like complete blood count, and electrolyte level, an increase in the serum activities of enzymes AST and ALT is used as an indirect biochemical indicator of symptomatic or asymptomatic hepatic tissue damage in toxic exposure and drug-induced toxicity [13,30]. In this study, there was a significant increase in the activities of AST and ALT in both groups treated with Benylin with codeine syrup (Figures 1 and 2). Necrosis of hepatocytes and moderate steatosis were also observed in the liver histology of these groups (Plate 6) indicating distortion of cytoarchitecture by the treatment. According to Lee [13], Real, et al. [29], and Ahmad, et al. [30], hepatoxicity induced by some drugs can occur from the conversion of drugs into reactive toxic metabolites and/or induction of immuno-allergic reactions. Codeine is a prodrug that is hepatically converted to morphine by CYPD26, a toxic metabolite [4]. It was noted that hepatic toxicity can result from an increased concentration of codeine metabolites due to the overdose-induced metabolism of more codeine (Crews, et al. 2021). Metabolites from hepatic metabolism are transported to the kidney for clearance. A suspected increase in plasma creatinine level, a marker of renal function [15] following treatment with Benylin-codeine syrup suggests the presence of renal impairment which may have resulted from overwhelming concentrations of toxic codeine metabolites. An increase in activities of AST, ALT, and liver injury may have occurred due to the conversion of more codeine reaching the liver to its reactive toxic metabolites and/or higher plasma concentration of codeine, morphine, and morphine-6-glucuronide due to impaired clearance. Abuse of codeine from repeated consumption of Benylin-codeine syrup has been shown in this study to induce hepatic injury and dysfunction, and impaired renal clearance that may have contributed to the alterations recorded in the blood.
The brain is made up of different areas that are connected by different nerve projections. Cortical areas (midbrain and frontal cortex), basal ganglia, and lateral portions of the cerebellum are the critical regions of the brain with dopaminergic connections working in circuitry to control behavior such as pleasure and addiction, and as well plan and coordinate movement [31]. Most drugs of abuse have been reported to target these brain areas controlling movement, emotions, motivations, and feelings of pleasure [10,13,21]. Studies have shown that opioids such as morphine can cause the nerve cells to release abnormally large amounts of neurotransmitters or prevent the normal recycling of these neurotransmitters thereby affecting both the structure and function of reward and affecting processing areas of the brain [32]. Brain enzymes, neurotransmitters, nerve cell make-ups, transmembrane receptors, and other macro-molecules are majorly composed of proteins and this study showed a significant decrease in brain total protein level in codeine-treated rats when compared to normal control. Reports from the studies of Tijani, et al [13] and Yunsung, et al. [10] revealed that disruption in underlying mechanisms involved in motor control and memory. And since these pathways are made of proteins (enzymes, nerve cells, and neurotransmitters), impairments reported in earlier studies could be linked to the decrease observed in brain total protein in this study (Figure 4). The significant decrease in the brain’s total protein level (Figure 4) could be due to nerve cell degeneration, membrane receptor derangement, and alteration in the recycling of neurotransmitters which are majorly made up of proteins. Hence, abuse of Benylin-codeine syrup would not only elicit behavioral and pscychotoxic effects but would also cause significant derangement of a brain protein that contributes largely to the functions of enzymes, nerve cells, and neurotransmitters.
In addition, histological observation of the brain showed fibrotic stroma with mild infiltrations by inflammatory cells (Plate 2-5), spanned cortical layer (Plates 3,4), necrosis of the cortical focal area (Plate 4), hyperplasia of the granular layer of the cerebellum, shrunk and hyalinized Purkinje cells (Plates 3-5). According to Stein and Thompson [33] and Snell [34], damage to the cortical areas can alter coordination and cognition function while injury to the cerebellum can delay initiation and decomposition of movements and as well cause gait and posture disturbance. Prolonged use of opioids such as morphine and heroin has been reported to cause hypersensitivity of dopaminergic receptors in basal ganglia and an imbalance in motor control [35]. Hyperplasia of the granular layer which was observed in the brain cells of mice treated with codeine has also been reported to lead to disruption of the smooth flow of neural information and further propagation of mossy fibers. Alterations in micro-anatomy of the brain areas of mice treated with benylin-containing codeine suggest the possibility of brain injury and neurodegeneration in humans consuming this syrup indiscriminately.
There are evidences that opioids such as codeine can be effective in the management of a variety of chronic pain and other medical conditions but this study showed that chronic or indiscriminate use (abuse) of Benylin-containing codeine syrup could induce hematological alteration, renal impairment, hepatocellular, lung and brain injuries with increased risk of cardiovascular disorders and other related complications. These effects are dose-dependent.
- Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1995 Mar;52(3):219-29. doi: 10.1001/archpsyc.1995.03950150051010. PMID: 7872850.
- Fields RD. New culprits in chronic pain. Sci Am. 2009 Nov;301(5):50-7. doi: 10.1038/scientificamerican1109-50. PMID: 19873904.
- Willis WD Jr. The somatosensory system, with emphasis on structures important for pain. Brain Res Rev. 2007 Oct;55(2):297-313. doi: 10.1016/j.brainresrev.2007.05.010. Epub 2007 Jun 12. PMID: 17604109.
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010 Jan 19;152(2):85-92. doi: 10.7326/0003-4819-152-2-201001190-00006. PMID: 20083827; PMCID: PMC3000551.
- Davis H, Baum C, Graham DJ. Indices of drug misuse for prescription drugs. Int J Addict. 1991 Jul;26(7):777-95. doi: 10.3109/10826089109058920. PMID: 1960000.
- World Health Organisation. Dextromethorphan PreReview Report, 35th Expert Committee on Drug Dependence (ECDD) Hammamet, Tunisia, 4-8 June 2012 Agenda item 5.1.
- Uwadiae E. Psychoactive substance use/abuse among students in Igbinedion University, Okada, Nigeria –New challenges. Nig J Gen Prac. 2011; 9: 31-34.
- Tijani AY, Salawu OA, John-Africa LB, Abubakar S, Chindo BA. Behavioural effects of Benylin-Codine in mice Nature & Science. 2012;10(4): 83-88.
- United Nations on Drugs and crime, UNDOC (2018) Drug Use in Nigeria World Drug Report 1-81.
- Nam Y, Shin EJ, Yang BK, Bach JH, Jeong JH, Chung YH, Park ES, Li Z, Kim KW, Kwon YB, Nabeshima T, Kim HC. Dextromethorphan-induced psychotoxic behaviors cause sexual dysfunction in male mice via stimulation of σ-1 receptors. Neurochem Int. 2012 Nov;61(6):913-22. doi: 10.1016/j.neuint.2012.01.025. Epub 2012 Feb 2. PMID: 22326744.
- Biose IJ, Oremosu AA, Gbotolorun SC, Adegoke AA, Akang NE, Bakare AA. The Effect of Dextromethorphan on the Testes of Adult Sprague-Dawley Rats. Afr J BioMed Res. 2012; 16: 115-123.
- Dighe AS, Dighe CA, Magar SD. Cytochrome Oxidase Enzyme- Its Role In Drug Metabolism- Review. Euro J Pharmaceu and Med. Res. 2018; 58: 241-243
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003; 374: 474
- Polsfon GR, Wallacce MS. Anallgesics agents in Rheumatic Disease. Kelly and Firestein’s Textbook of Rheumatology. 10th Edition, Elseiver Inc, USA. 1075-1095. 2017.
- Penson RT, Joel SP, Roberts M, Gloyne A, Beckwith S, Slevin ML. The bioavailability and pharmacokinetics of subcutaneous, nebulized and oral morphine-6-glucuronide. Br J Clin Pharmacol. 2002 Apr;53(4):347-54. doi: 10.1046/j.1365-2125.2002.01554.x. PMID: 11966664; PMCID: PMC1874271.
- Chiang J. Liver Physiology: metabolism and detoxification. Pathobiology of Human Disease. 2014; 1770-1782.
- Gao B. Basic liver immunology. Cell Mol Immunol. 2016 May;13(3):265-6. doi: 10.1038/cmi.2016.09. Epub 2016 Apr 4. PMID: 27041634; PMCID: PMC4856812.
- de Montalembert M. (2008). Management of sickle cell disease. Br med J. 337:626
- Mazza MG, Rossetti A, Clerici MA. Review of Neutrophil-Lymphocyte, Monocyte-Lymphocyte, and PlateletLymphocyte Ratios Use in Psychiatric Disorders. World J Depress Anxiety. 2018; 11: 1002.
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998 Mar 15;83(1-2):63-9. doi: 10.1016/s0165-5728(97)00222-1. PMID: 9610674.
- Weber RJ, Gomez-Flores R, Smith JE, Martin TJ. Immune, neuroendocrine, and somatic alterations in animal models of human heroin abuse. J Neuroimmunol. 2004 Feb;147(1-2):134-7. doi: 10.1016/j.jneuroim.2003.10.029. PMID: 14741445.
- Stephanou A, Fitzharris P, Knight RA, Lightman SL. Characteristics and kinetics of proopiomelanocortin mRNA expression by human leucocytes. Brain Behav Immun. 1991 Dec;5(4):319-27. doi: 10.1016/0889-1591(91)90027-8. PMID: 1685686.
- Vallejo R, De Leon-Casasola O, Benyamin R. Opoid therapy and immunosuppression. Amer J Therapeut. 2004; 11: 354-365.
- Ataseven A, Ugur Bilgin A. Effects of isotretinoin on the platelet counts and the mean platelet volume in patients with acne vulgaris. ScientificWorldJournal. 2014 Jan 29;2014:156464. doi: 10.1155/2014/156464. PMID: 24605049; PMCID: PMC3925551.
- Al-Bairmany YS, Aqabi AS, Al-Hasnawi FH, Al-Aawad AS. Usefulness of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as a predictor of disease-free survival in breast cancer: A cross-sectional study F1000Research. 2019; 8: 306.
- Di Vito C, Navone SE, Marfia G, Abdel Hadi L, Mancuso ME, Pecci A, Crisà FM, Berno V, Rampini P, Campanella R, Riboni L. Platelets from glioblastoma patients promote angiogenesis of tumor endothelial cells and exhibit increased VEGF content and release. Platelets. 2017 Sep;28(6):585-594. doi: 10.1080/09537104.2016.1247208. Epub 2016 Nov 29. PMID: 27897101.
- Guetl K, Raggam RB, Muster V, Gressenberger P, Vujic J, Avian A, Hafner F, Wehrschuetz M, Brodmann M, Gary T. The White Blood Cell Count to Mean Platelet Volume Ratio for the Prediction of Chronic Limb-Threatening Ischemia in Lower Extremity Artery Disease. J Clin Med. 2019 Oct 2;8(10):1593. doi: 10.3390/jcm8101593. PMID: 31581728; PMCID: PMC6832925.
- Anggara R, Warli SM, Siregar GP. Correlation of Platelet-to-Lymphocyte Ratio PLR, Neutrophil-to-Lymphocyte Ratio NLR to Gleason Score in Prostate Cancer Patients at Haji Adam Malik Medan General Hospital. International Journal of Medical Science and Clinical Invention. 2018; 505: 3824-3826.
- Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf. 2019 Mar;42(3):365-387. doi: 10.1007/s40264-018-0743-2. PMID: 30343418.
- Ahmad J, Reddy KR, Tillmann HL, Hayashi PH, Chalasani N, Fontana RJ, Navarro VJ, Stolz A, Barnhart H, Cloherty GA, Hoofnagle JH. Importance of Hepatitis C Virus RNA Testing in Patients with Suspected Drug-Induced Liver Injury. Dig Dis Sci. 2019 Sep;64(9):2645-2652. doi: 10.1007/s10620-019-05591-w. Epub 2019 Mar 29. PMID: 30927209; PMCID: PMC6706305.
- Lash ML. Neurophysiological Basis of Movement, 2nd ed. Human Kinetics. 2008.
- Younger JW, Chu LF, D'Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011 Aug;152(8):1803-1810. doi: 10.1016/j.pain.2011.03.028. Epub 2011 Apr 30. PMID: 21531077; PMCID: PMC3138838.
- Stein RB, Thompson AK. Muscle reflexes in motion: how, what, and why? Exerc Sport Sci Rev. 2006 Oct;34(4):145-53. doi: 10.1249/01.jes.0000240024.37996.e5. PMID: 17031251.
- Snell RS. Clinical Neuroanatomy 6th Edition. Lippincott Williams and Wilkins. 2006; 219-305.
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992 Sep;53(5):447-57. doi: 10.15288/jsa.1992.53.447. PMID: 1405637.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
