Advances in Toxicology and Toxic Effects
In-depth Characterization of “Eco-Friendly” EN AW 6026 LF Aluminium Alloy, with Intentionally Reduced Content of Pb and Sn, in Application for Direct Contact with Foods
Sinagra Ciro1*, Bravaccino Francesco1, Di Betta Giorgio2, Cazzago Gianfranco2, Cusan Claudia3, Sguazzin Alessia3, Greco Vincenza4 and Bellucci Francesco5
2Eural Gnutti Spa, Rovato, Brescia 25038, Italy
3S&C BEST Srl, Portogruaro 30026, Venice, Italy
4UOC Farmacia, AORN Cardarelli, 81031, Napoli, Italy
5CRdC Tecnologie Scarl Via Nuova Agnano 11, 80125 Napoli, Italy
Cite this as
Ciro S, Francesco B, Giorgio DB, Gianfranco C, Claudia C, Alessia S, et al. In-depth Characterization of “Eco-Friendly” EN AW 6026 LF Aluminium Alloy, with Intentionally Reduced Content of Pb and Sn, in Application for Direct Contact with Foods. Adv Toxicol Toxic Effects. 2025; 9(1): 001-008. Available from: 10.17352/atte.000020Copyright
© 2025 Ciro S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.In order to enhance the mechanical processing of aluminium alloys, the utilisation of aluminium alloys containing lead (Pb) and tin (Sn) has been adopted in many industrial applications. In order to improve the environmental impact and to open up further additional markets, a new aluminium alloy, EN AW 6026LF, has been developed by substituting Pb and Sn with Bi, which has been added as an alloying agent at a level of approximately 1%. Consequently, this study aims to provide a comprehensive evaluation of the potential of EN AW 6026LF for utilisation in food machinery and packaging applications. The experimental analyses conducted include microstructural characterisation, corrosion resistance on the bare and anodised alloy, and Bi migration tests in various aqueous simulants. The toxicity evaluations have shown that the release of Bi remains within safe limits under various conditions, which should ensure consumer safety. This comprehensive assessment underscores the versatility, sustainability, and regulatory compliance of EN AW 6026LF, which could be considered a viable alternative to traditional lead-containing alloys in environmentally sensitive and direct food contact applications.
Abbreviations
bw: Body Weight; ECHA: European Chemical Agency; EFSA: European Food Safety Authority; FCA: Food Contact Additives; kg: kilogram; LD50: Lethal Dose, 50%; LLNA: Local Lymph Node Assay; NOAEL: No Observed Adverse Effect Level; OECD: Organisation for Economic Co-operation and Development; PoD: Point of Departure; SCCS: Scientific Committee on Consumer Safety; TDI: Tolerable Daily Intake; UF: Uncertainty Factor; SEM: Scanning Electron Microscope; GD-OES: Glow Discharge – Optical Emission Spectroscopy; OCP: Open Circuit Potential; icorr: Corrosion Current Density; Ecorr: Corrosion Potential; Rp: Polarisation Resistance; ICP-MS: Induced Coupled Plasma – Mass Spectroscopy; S/V: Surface/Volume ratio; MPPO: Modified Polyphenylene Oxide; RoHS: Restriction of Hazardous Substances Directive; ELV: End-of-Life vehicles; REACH: Registration, Evaluation, Authorisation and Restriction of Chemicals.
Introduction
The 6026LF alloy is anticipated to be utilised in the domain of machinery and equipment for food production, including mechanical components and rollers, as well as for packaging components in the field of cosmetics. Prior to the advent of 6026LF, the alloys that were employed for such applications, primarily for free cutting operations, were 6061, 6082, and, in select instances, 6063 or equivalent. The utilisation of these alloys during machining processes invariably results in the formation of undesirable phenomena such as long curls and chips, which in turn give rise to quality issues on the surface of the finished components and significantly extended production cycles. In contrast, the 6026LF alloy, distinguished by its incorporation of bismuth and the implementation of specific heat treatments, exhibits a remarkable capacity for chip-breaking, thereby markedly reducing cycle times and enhancing surface finish. Moreover, in the current regulatory environment, lead-free high-processability alloys are subject to an even more challenging regulatory framework following the approval of the European Commission’s Delegated Regulation (EU) 2024/197 of 19 October 2 023, published on 5 January 2024, amends Regulation (EC) No 1272/2008 with regard to the harmonised classification and labelling of certain substances, including lead. This will be accompanied by the introduction of even more stringent regulations, which are currently being reassessed. These include the Restriction of Hazardous Substances Directive (RoHS), the End of Life Vehicle Directive (ELV), and most importantly, the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) Directive. The newly approved regulation is a concrete cornerstone for the protection of health and the environment, for which it was worth investing heavily in research and development for a more sustainable material, ranging from engineering purposes up to direct food contact applications. In light of the above restrictions, the Laminazione Sottile group and Eural Gnutti Spa addressed an investigation on modified Al alloy 6026LF with the aim of its potential application in the food market. Alloy 6026LF (lead-free), was first developed for use in automotive brake systems, in response to the high demand for more critical automotive applications, in which the Pb and Sn content has been reduced to trace levels, which allows it to comply with the stringent European Union regulations, including REACH and RoHS directives. The product is registered with the International Organization for Standardization (ISO), Euronorm (EN), and American Society for Testing and Materials (ASTM) standards and has been approved and widely used by major industries, including automotive, aerospace, pneumatics, hydraulics, valves, and electrical and electronic components. This makes it a more sustainable alloy in both manufacturing (Sn 0.05% wt max) and recycling, reflecting the limitations inherent to REACH regarding Pb content (max. 0.1%) while maintaining its basic characteristics. The latest studies have shown that alloy 6026LF is suitable for use in contact with food due to its complete non-toxicity and compliance with the recent Delegated Regulation (EU) N. 197/2024, which will open up further markets such as food machinery, packaging for cosmetics, and more.
Moreover, its remarkable ability to undergo hard or even merely decorative anodising renders the alloy highly versatile and particularly well-suited for applications where a high corrosion response is required [1,2]. Finally, it is also extensively employed in situations where excellent welding capabilities are required and as a substitute for brass, where it exhibits comparable workability and mechanical characteristics at a third of the weight.
Experimental
Sample preparation
The experimentation was carried out on bars 6026LF aluminium alloy, drawn in 21.5 mm diameter rods and aged in the T9 state (artificial ageing following solution heat treatment and subsequent cold working to enhance product strength), whose chemical composition, measured on polished bars by Spark-OES technique, is given below in Table 1:
Each bar was divided into many slices and characterised by using the flat circular face polished with 1 µm diamond suspension; both plain and anodised samples have been investigated. The anodized slices were prepared by degreasing the bars with an alkaline degreaser, followed by glazing for 20 seconds, and anodized in Sulphuric Acid at 180-200 g/l, 12-15 V at room temperature. Finally, the oxide layer was cold-fixed for 10-15 minutes in a Nickel Fluoride bath.
Results and discussion
SEM analysis and description of secondary phases (precipitates), crystallographic structure, electrochemical and corrosion behaviour, Bi release assessment test, toxicological evaluation, and Tolerable Daily Intake (TDI) derivation of Bi were addressed in this paper to assess the use of the alloy as suitable material for the food contact market
SEM analysis and description of secondary phases
A half-centimetre slice was taken from the rods in order to embed it in conductive thermosetting resin and polish one of the two circular mirror surfaces with a 1 µm finish. This surface was then observed under a scanning electron microscope and the secondary phases were analysed by using an EDS probe. Below are some images with their elemental spectroscopy data reported (Table 2).
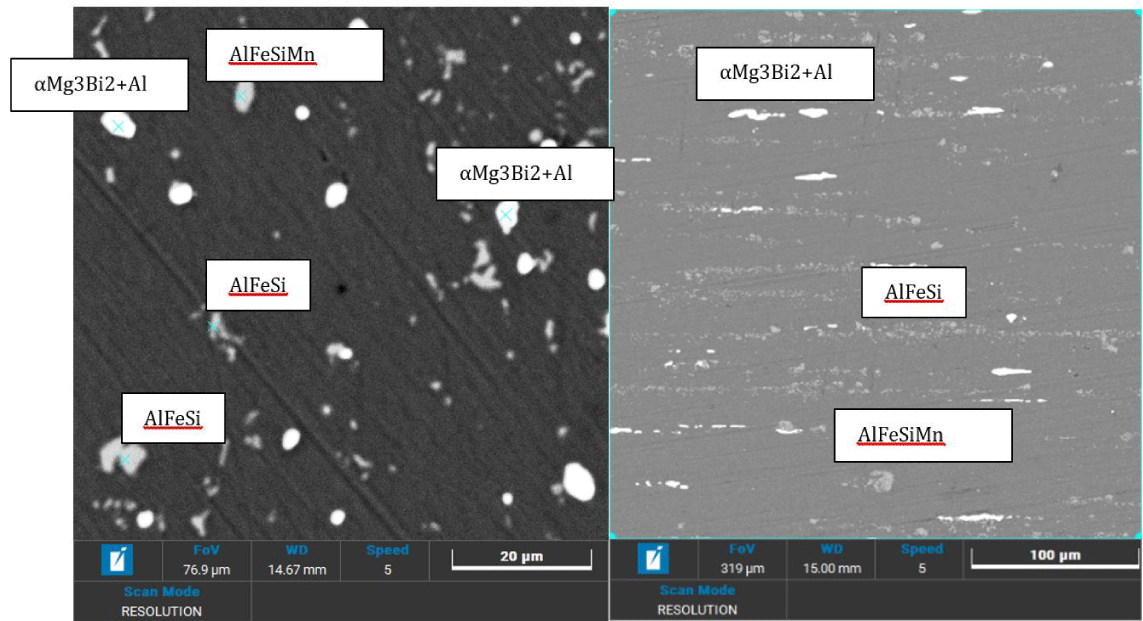
The bar shows a high abundance of Bismuth- and Magnesium-rich precipitates (as Magnesium Bismuthite, Mg3Bi2) in globular form, accompanied by AlFeSi and AlFeSiMn intermetallics compound (Figure 1). The remaining characteristic alloying elements, such as Zn, Cu, Cr, Ti, Ga, Sn, and Pb are all in solid solution at the concentrations observed and do not exhibit characteristic molecular precipitates.
SEM results on the anodized samples were reported in Figures 2,3, while the EDS data were summarized in Table 3.
No presence of Bi or other elements like Pb or Sn can be found on the outermost layers. The oxide present on the polished and anodised face is very porous and with circular grooves; sometimes it traces the outline of the intermetallics previously present in the metal where the oxide did not have a chance to form, leaving grooves with irregular (dark) shapes.
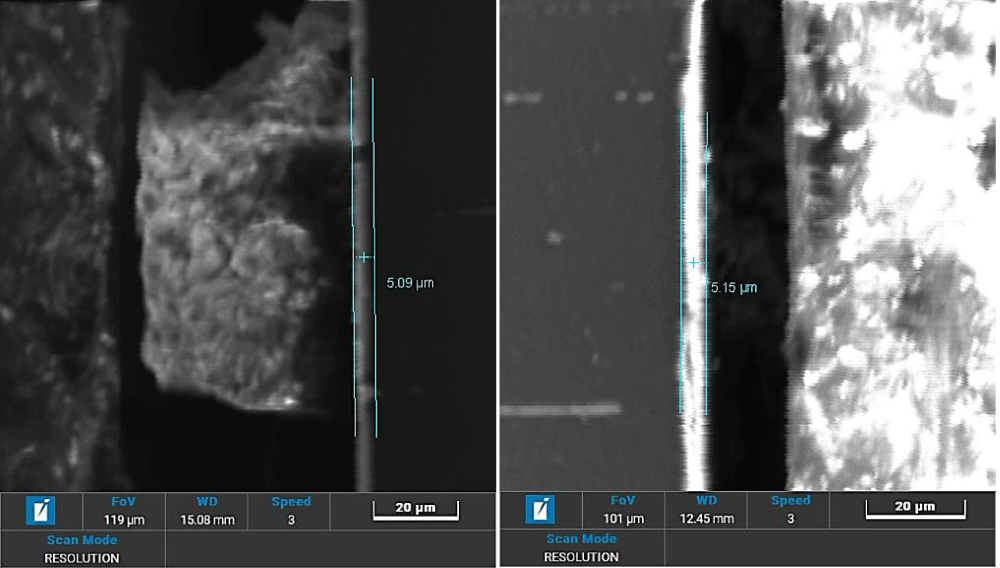
Visible on the sample in the longitudinal section is the very compact oxide layer formed by the anodising process, the thickness of which, measured with both Scanning Electron Microscopy (SEM, Figure 3) and Glow Discharge – Optical Emission Spectroscopy (GD-OES) techniques, are between 5 and 9 microns (also considering the diffusion of oxygen in the metal, which is very clearly visible on the GD-OES Profilometric spectrum, “fuchsia” curve, Figure 4). No presence of Bi or other elements like Pb or Sn can be found on the outermost layers.
Metallographic preparation and crystallographic structure
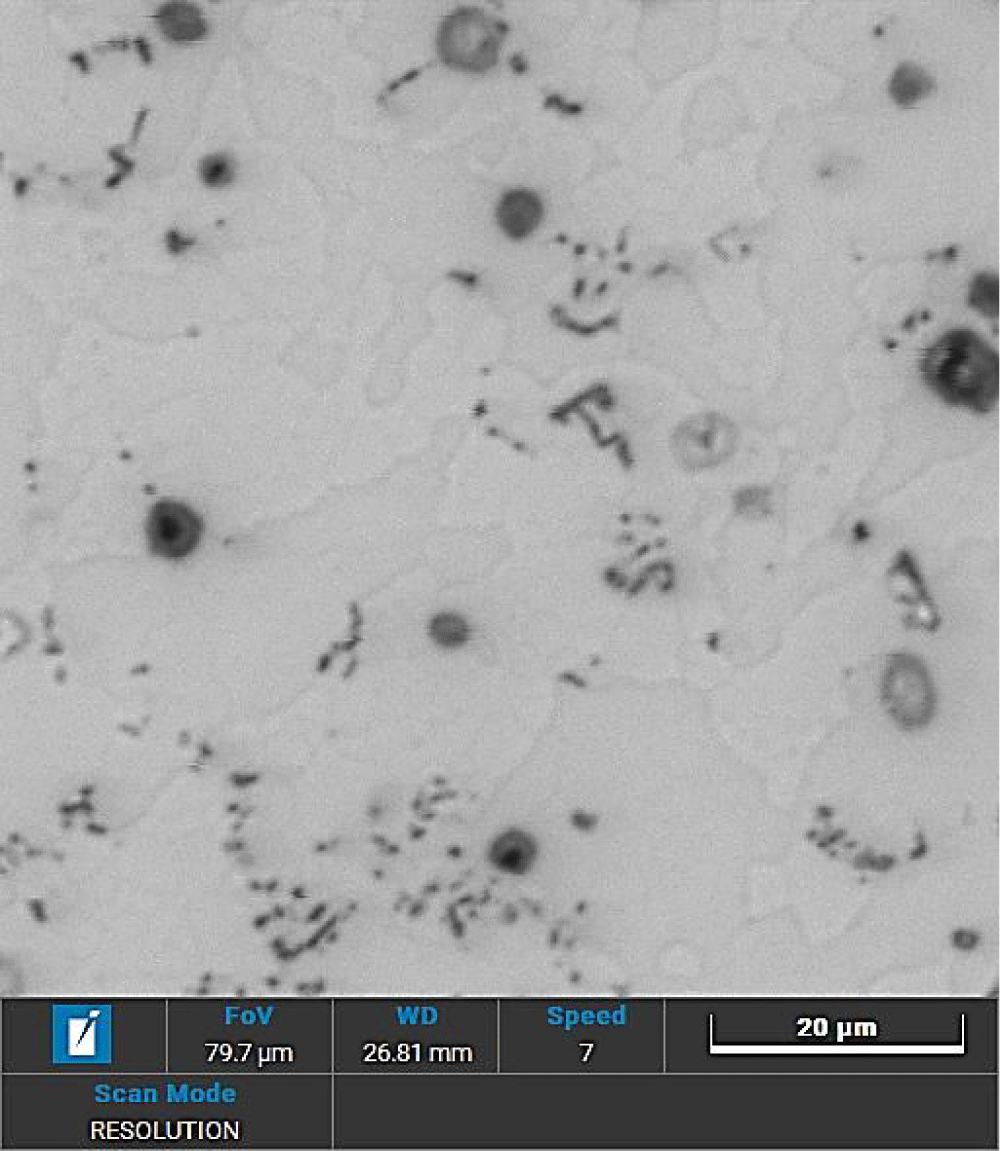
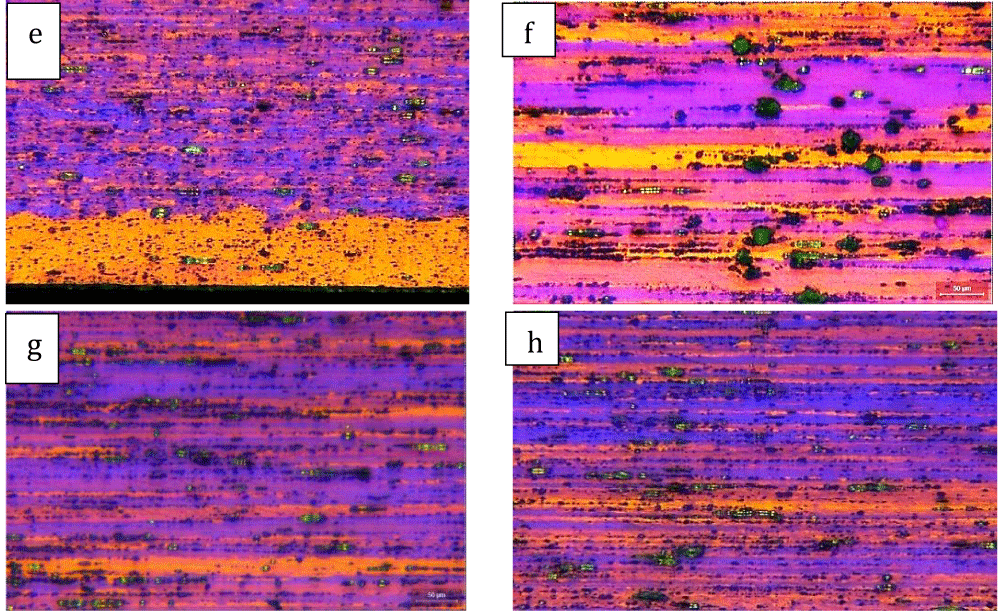
The polished plain slices were etched in Barker’s reagent @ 20V for 10 seconds and observed under a polarised tint-sensitive plate in Light Optical Microscopy (LOM) to reveal the crystallographic structure. Here some details of the bars, observed on the central core and side areas are reported, in front and side sections (Figures 5,6).
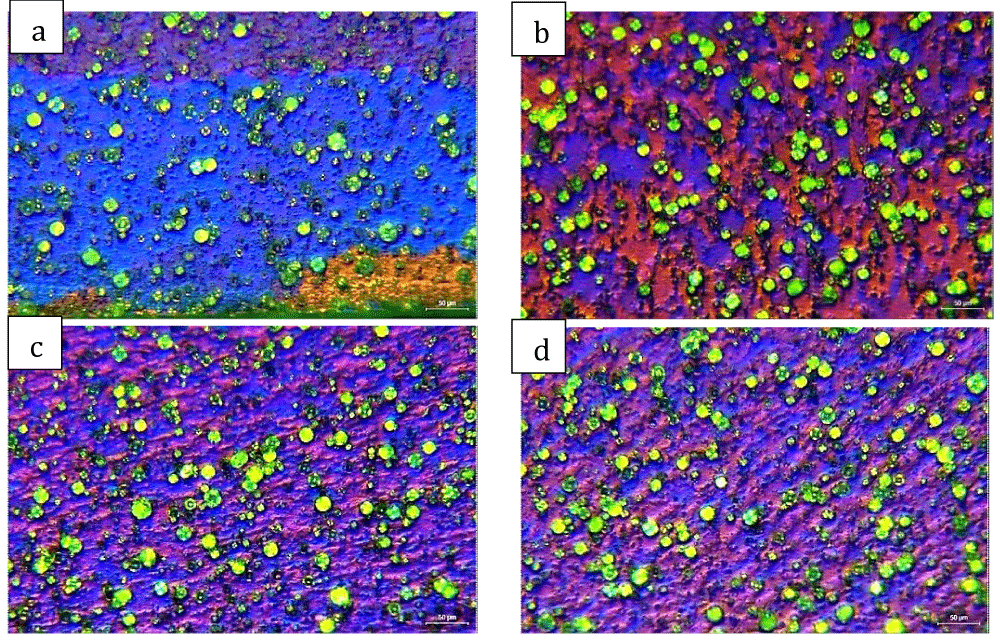
The structure of the bars is mostly hardened, with fine elongated grains in the drawing direction, filled with Bi precipitates (green spheres on the front view and black strokes on the side view, Figures 5,6); some growth can be seen on the edge of the bars (Figure 5a,6e), due especially to the bar-drawing process which, even though the presence of lubrication in the process, the contact temperature reached on the surface of the bar can re-crystallize the structure on the spot.
Electrochemical and corrosion behaviour
The electrochemical and corrosion behaviour of the alloy EN AW 6026 LF was carried out by cutting a small slice of half a centimetre from the bar; the part was then encased in epoxy resin by exposing one of the two circular surfaces and firmly connecting the other to an electric wire with a conductive resin to create an electrical contact with the part, after roughing the surface to remove cutting residue and improve the contact between wire and part. Following the embedding and curing of the epoxy resin, the surface not coated by the resin was ground and polished to a mirror finish at 0.03 µm.
Electrochemical tests were conducted using a 595 ASTM aerated Corrosion Cell, according to ASTM G59-23 [3], using a 3.5 % wt solution of NaCl continuously aerated with double purity air. The configuration used is that of a 3-electrode cell using the sample as the working electrode, a platinum electrode as the counter electrode, and a Saturated Calomel Electrode (SCE, +0.248 V vs. SHE at 20 °C) as the reference electrode. Cyclic polarisation scans were performed by bringing the system to a maximum through current density of 100mA/cm² and returning the over-potential to Open Circuit Potential (OCP), at room temperature and then at 50 °C via a heating plate placed under the ampoule [3-5]. The latter temperature was chosen in order to simulate the operating conditions of the machinery processes.
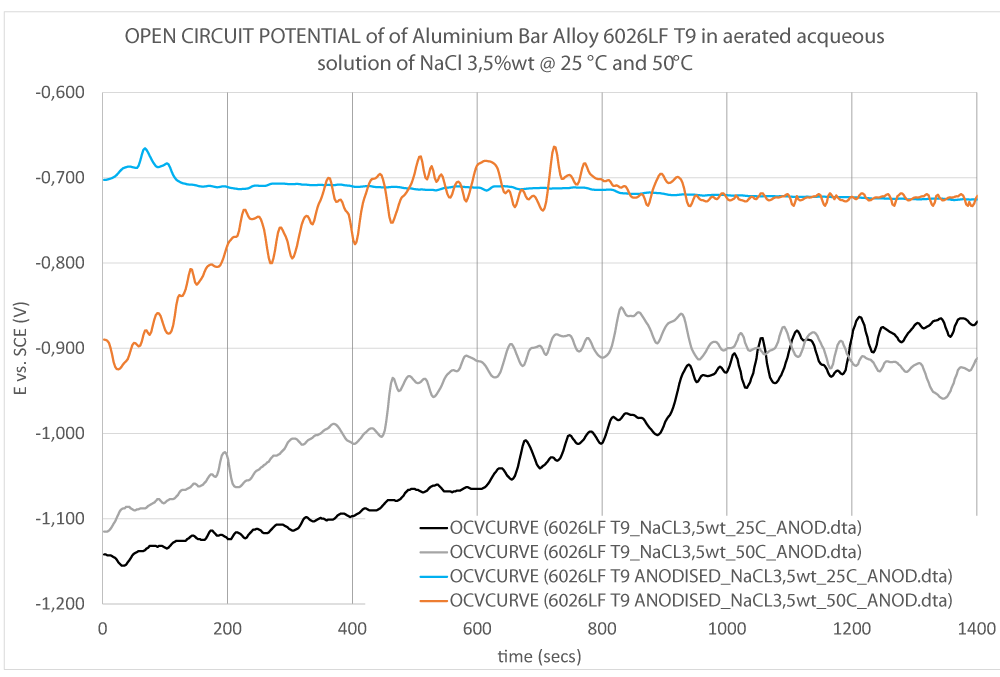
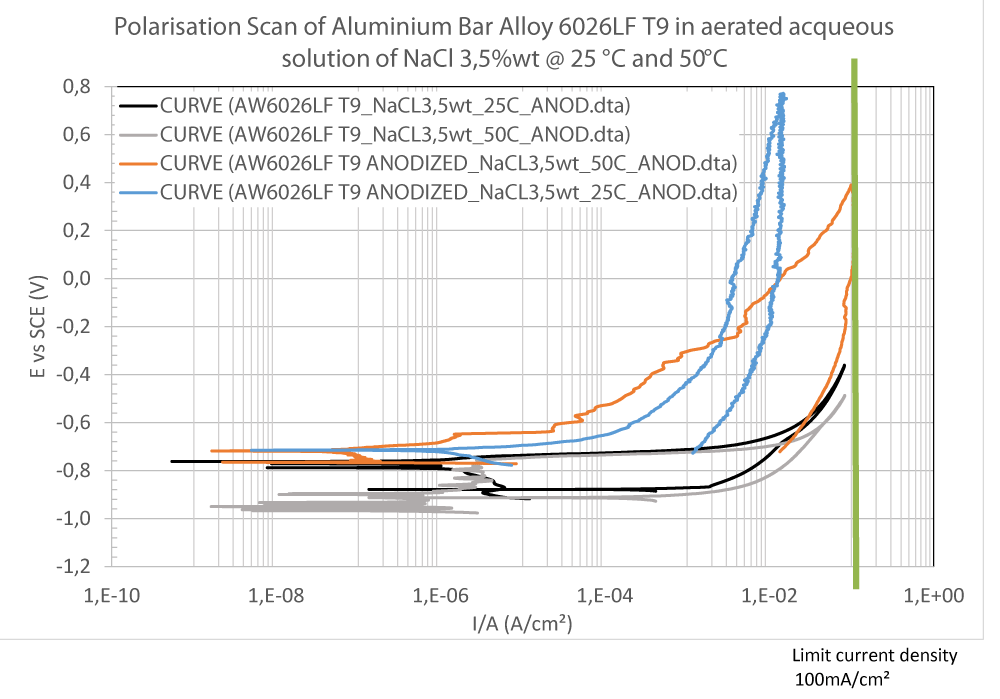
Experimental data of OCP and Evans curves of the cyclic anodic branch are shown (Figures 7,8).
As can be seen in Figure 8, no passivity is observed for the bare alloy as expected (Black and Grey curve in Figure 8); whereas a very strong passivity is visible on the anodised alloys (Orange and Blue curves in Figure 8). However, a shift of the curve at 50 °C (Orange curve in Figure 8) was observed suggesting a more susceptibility of the alloy to corrosion at high temperatures.
From Figure 8, an estimate of the corrosion rate of both bare and anodised samples in the different conditions was evaluated [4] and reported in the following Table 4:
Results reported above suggest that in a potential food market analysis, the alloy EN AW 6026 LF must be used in the anodised state rather than the bare.
Bi-release assessment test from aluminium alloy
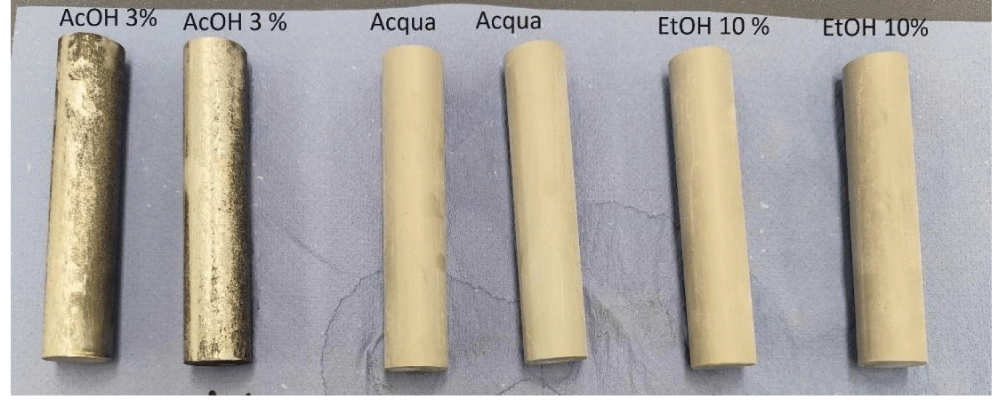
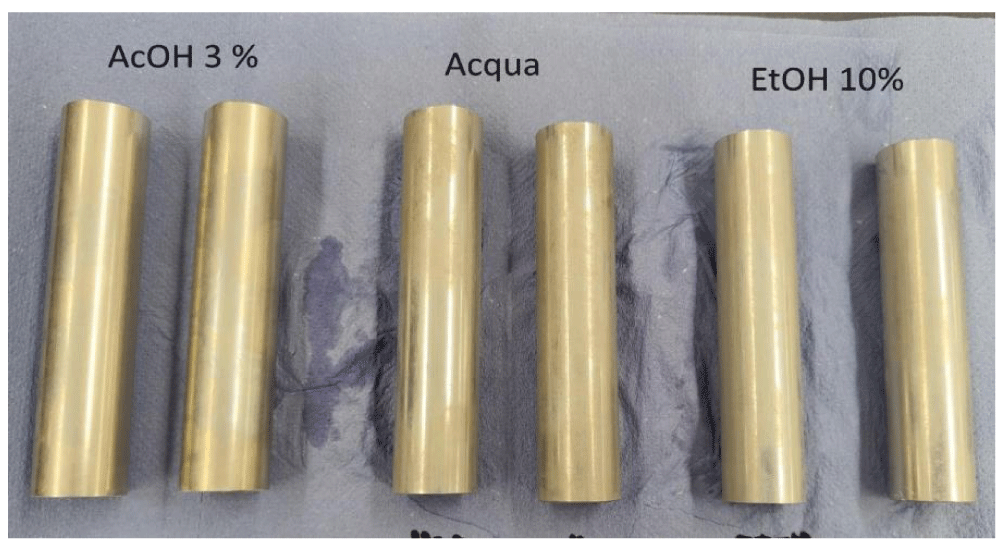
Bi determination after contact 10 days 60°C: In order to simulate the worst-case conditions of prolonged contact of alloy EN AW 6026 LF and food, as required by Regulation 10/2011, an analysis of Bi migration was carried out on bare alloy rather than the anodised one, after 10 days at 60 °C in: (i) 3% acetic acid, (ii) in water, (iii) in 10% ethanol, and (iv) in oil. Samples of 82.9 cm2 were exposed to 138 mL of simulant (S/V ratio=0.6) [6] throughout, as required by Regulation 10/2011. Bi-migration data in all the above conditions are reported in Table 5.
The visual aspect of all samples is shown in Figure 9. Sample exposed to acetic acid 3% appear attached, whereas only opacification was observed after contact with water and 10% ethanol (Figure 9).
No changes were observed after contact with the oil.
Bi determination after contact 4h at 100 °C: The analysis was carried out following a 4-hour contact at 100 °C in aqueous simulants, acetic acid 3% equivalent to a 1-hour contact at baking temperatures [6] (Table 6).
After contact with Acetic acid 3%, Water, and Ethanol 10% for 4 hours at 100 °C, slight acidification is observed.
No changes were observed after contact with the oil (Figure 10).
Determination of Bi after contact 1h 175 °C: The analysis was conducted after a 1-hour contact at 175 °C with oil simulant. Determination of Bi in oil was performed after an acid mineralisation process and subsequent ICP-MS analysis [6] (Table 7).
No changes were observed after contact 1 hour at 175 °C (Figure 11).
Bi determination after contact 1h 200 °C: For temperatures above 175 °C, simulant E (Tenax, as required by Regulation 10/2011) was used as an oil substitute for the analysis of bismuth migration in MPPO. The determination of bismuth in MPPO was performed after an acid extraction process and subsequent analysis in ICP-MS [6] (Table 8).
No changes are observed after contact with MPPO for 1 hour at 200 °C.
According to the report ‘Toxicological Evaluation and Tolerable Daily Intake Derivation of Bi’ and based on available toxicological data, Bi is not mutagenic, carcinogenic, or toxic to reproduction. Several studies indicate that insoluble Bi compounds show low toxicity, while soluble organic compounds, such as Bismuth citrate, show higher toxicity. This increased toxicity is probably due to the increased absorption of soluble bismuth salts in the human body after ingestion.
The oral Tolerable Daily Intake (sTDI) was calculated using the most reliable and conservative toxicological data in the SCCS opinion for bismuth citrate, a water-soluble salt of Bi.
The sTDI is set at 0.08 mg/kg body weight per day. This value was determined according to the FCA 2020 guidelines [7] and corresponds to an oral intake level of 4.8 mg per person per day for an adult of 60 kg body weight, thus corresponding to a specific migration value of 4.8 mg/kg food. The migration values found in the migration tests performed were always lower than the specific migration limit calculated from the sTDI and therefore, based on the analyses performed, no critical issues related to the release of Bi were reported.
Toxicological evaluation and tolerable daily intake derivation of Bi
In the present report, the self-derived oral Tolerable Daily Intake (TDI) is derived from the most reliable and conservative toxicological data reported in SCCS opinion for Bismuth Citrate, which is a water-soluble Bi salt. The self-derived TDI is established at 0.08 mg/kg bw/day. This value was derived according to FCA 2020 guidelines and corresponds to an oral safe level of 4.8 mg/person/day, for an adult of 60 kg standard body weight [8].
Toxicological profile of Bi:
General information:
Bi atom is a pnictogen and a metal atom [9]. It is the most metallic and the least abundant of the elements in the nitrogen group [10].
In nature, Bi can be found as a free metal or in combination forms such as bismite (bismite oxide) and bismuthite (bismuth sulfide), which are frequently found in lead, copper, and tin dioxide sulfide ores [10].
Humans are mostly exposed to Bi through their diet because it is a naturally occurring element; typical dietary intake ranges from 5 to 20 μg/day (0.07 to 0.29 μg/kg bw/day), assuming a body weight of 70 kg [11] .
Bi exists in trivalent and pentavalent oxidation states (the trivalent being more abundant and stable) and forms soluble and insoluble, organic and inorganic salts. In general, insoluble salts such as bismuth citrate and subcarbonate are of low toxicity; neurotoxic effects are associated predominantly after ingestion of excessive amounts of lipid-soluble organic salts, e.g. bismuth subgallate (from 3 to 20 g per day); renal toxicity is associated with high exposures to water-soluble organic compounds, e.g. bismuth sodium triglycollamate, bismuth subsalicylate, and bismuth sodium tartrate [9], due to accumulation in kidney as reported for other soluble bismuth organic compounds like bismuth sodium tartrate and tripotassium dicitratobismuthate [12-14]. As an example, overdosing on bismuth subcitrate, used to treat peptic ulcers and H. pylori infections, has been reported to result in serious, but reversible, nephrotoxicity in humans. Still, little is known about the nature of renal damage induced by bismuth [15].
In general, Bi is considered the safest heavy metal due to the low solubility of its various salts [9]. Due to its low toxic potential resulting from low bioavailability (and thus systemic absorption) following oral dosing, the toxicity database for bismuth is limited [11].
The present evaluation of Bi was conducted in line with the European Food Safety Authority’s (EFSA) guidelines for the assessment of materials in contact with food. Specifically, the literature was reviewed to determine the potential for carcinogenicity, genotoxicity and mutagenicity, developmental and reproductive toxicity, sub-chronic and chronic toxicity (repeated dose studies), toxicokinetics and toxicodynamics, and epidemiology (humans). Several public resources, including eChemPortal, the Organisation for Economic Co-operation and Development (OECD) Existing Chemicals Database, European Chemical Agency (ECHA), EFSA, PubChem, PubMed, and the Scientific Committee on Consumer Safety (SCCS), were used to achieve this. The search was conducted using keywords such as “Bismuth” and “CAS 7440-69-9” together with terms like “toxicity,” “toxicological studies,” “safe level,” “safe limit,” and so forth [16].
Animal toxicological data: Bi is poorly absorbed via oral route (1% of Bi), it distributes and accumulates predominantly in the kidneys of rats [8,17].
Bi-metal is not acutely toxic via the oral route (oral, rat, Lethal dose, 50% (LD50) above 2000 mg/kg bw), and is not considered to be irritating to the skin nor to the eye. It is also expected to not be sensitizing according to a Local lymph node assay (LLNA) study performed on bismuth salt, bismuth hydroxide nitrate oxide [17].
Systemic toxicity was investigated through the evaluation of three studies gathered in public literature: Bi alone did not cause any significant changes in clinical signs at the highest dose tested in rats after 28 days of exposure (OECD Guideline 407, No Observed Adverse Effect Level (NOAEL) equal to 1000 mg/kg bw/day). Additionally, the absence of adverse effects occurred after 90 days of exposure to bismuth subnitrate in rats (OECD Guideline 408, NOAEL equal to 1000 mg/kg bw/day) [17].
On the other hand, bismuth citrate caused ingesta-filled caeca and nasal histopathology changes in the mid and high-dose groups of rats orally exposed according to OECD Guideline 408 for 90 days. A NOAEL equal to 16 mg of bismuth per kg bw/day was established (corresponding to mg Bismuth citrate per kg bw/day) for the observed effects [9].
As for mutagenicity and genotoxicity, data found in the literature revealed that no information concerning the genetic toxicity of bismuth metal is available, nevertheless, several bismuth salts tested negative in in vitro assays (mammalian cell gene mutation assay, in vitro mammalian chromosome aberration test, sister chromatid exchange assay in mammalian cells, bacterial reverse mutation assay). Because soluble bismuth compounds are not mutagenic, it can be considered that bismuth metal as a poorly soluble substance is not mutagenic nor genotoxic [17].
Carcinogenicity data on bismuth metal are not available. Some bismuth compounds have been studied in long-term studies with rats and mice and none of the studies reported tumor induction by bismuth compounds [9].
As for fertility and development, the only observation that may raise some concern was found in the testicles of male rats following intraperitoneal exposure: the impact of bismuth on Leydig cells in Wistar rats was investigated using auto-metallography. The rats were treated with a high dose of bismuth subnitrate, which was found to damage Leydig cells and reduce serum testosterone levels, suggesting a potential risk of bismuth to male reproduction [12]. However, SCCS came to the conclusion that these studies could not be used to draw conclusions about the risk assessment of male fertility and reproduction with regard to bismuth citrate or other bismuth species generated following oral absorption [9].
Self-derivation of the Tolerable Daily Intake (TDI): No oral safe level for bismuth has been established by any international organization or regulation, therefore, the TDI has been self-derived, according to the Food Contact Additives (FCA), 2020 guideline approach. The selected Point of Departure (PoD) is the same NOAEL chosen in the SCCS, 2013 opinion for the safety evaluation of bismuth citrate in cosmetic products [9]. The choice of using toxicological data from a salt instead of the metal relies on the fact that bismuth alone is even less absorbed than its salts, meaning that the toxicological studies performed on the metal alone could not be predictive of the actual toxicological potential of overall bismuth and its salts. Starting from the NOAEL equal to 16 mg/kg bw/day and by applying the Uncertainty Factors (UF) described by FCA, 2020 (100 for inter- and intraspecies and 2 for subchronic study duration) the self-derived TDI is calculated equal to 0.08 mg/kg bw/day [9]. The safe level calculated for a person of 60 kg body weight is, therefore, equal to 4.8 mg/day [9].
Conclusion
Results obtained in this paper suggest a potential use of the anodised aluminium alloy EN AW 6026 LF in the food market. Furthermore, toxicological data of Bi obtained on the most active bare surface alloy, have shown that the Bi release in food simulant solutions, according to European Regulation 10/2011, is negligible. This suggests that the anodised one must exhibit an even superior behaviour when exposed to food. From the toxicological evaluations, Bi is not mutagenic, not a carcinogen, and not toxic for reproduction. Several data are available, indicating that insoluble Bi compounds are related to low toxicity, whereas soluble Bi organic compounds such as bismuth subcitrate are associated with higher toxicity, probably due to the enhanced uptake of soluble Bi salts in human bodies after ingestion.
Author contributions
Conceptualization: Sinagra Ciro, Di Betta Giorgio, Bellucci Francesco;
Methodology: Bravaccino Francesco, Cusan Claudia and Sguazzin Alessia;
Validation: Sinagra Ciro, Cusan Claudia, Bellucci Francesco;
Toxicological analysis: Cusan Claudia, Sguazzin Alessia, Greco Vincenza;
Electrochemical and SEM investigations: Bravaccino Francesco;
Writing—original draft preparation: Bravaccino Francesco, Cazzago Gianfranco, Di Betta Giorgio.;
Writing—review and editing: Bravaccino Francesco;
Visualization: Bravaccino Francesco, Cusan Claudia;
Supervision: Sinagra Ciro, Bellucci Francesco;
All authors have read and agreed to the published version of the manuscript.
Data availability statement: The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of interest: The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
- Kaufman JG. Corrosion of Aluminum and Aluminum Alloys. Corrosion & materials [Internet]. 2005;95–124. Available from: https://doi.org/10.31399/asm.hb.v13b.a0003815
- Bardal E. Corrosion and protection. London: Springer-Verlag. 2004;68. Available from: https://www.scirp.org/reference/referencespapers?referenceid=134771
- ASTM G59. Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. 2023. Available from: https://www.astm.org/g0059-97r20.html
- Roberge PR. Handbook of Corrosion Engineering. 3rd ed. New York: McGraw-Hill Education; 2018. Wisdom J, Kelechi D, Ugochukwu J, editors. IRJET. 5:121. Available from: https://dl.icdst.org/pdfs/files/441d337b7410198db6d96e61a6716302.pdf
- ASTM International. ASTM G5-14(2021) Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements. Available from: https://www.astm.org/g0005-14r21.html
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Text with EEA relevance. Current consolidated version: 31/08/2023. Available from: http://data.europa.eu/eli/reg/2011/10/oj
- FCA. Guidelines on Risk Assessment of Non-Listed Substances (NLS) and Non-Intentionally Added Substances (NIAS) under the Requirement of Article 3 of the Framework Regulation (EC) 1935/2004. 2020. Available from: https://fca.cefic.org/wp-content/uploads/2021/02/FCA_Risk_Assessment_Guidelines_v30-1.pdf
- SCCS. Opinion on Bismuth citrate. 2013. Available from: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_147.pdf
- PubChem. PubChem Compound Summary for CID 5359367, Bismuth. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Bismuth
- Sanderson RT. Bismuth | Properties, Uses, Symbol, & Facts. Encyclopedia Britannica. 2025. Available from: https://www.britannica.com/science/bismuth
- Poddalgoda D, Hays SM, Nong A. Derivation of biomonitoring equivalents (BE values) for bismuth. Regulatory Toxicology and Pharmacology. 2020;114:104672. Available from: https://doi.org/10.1016/j.yrtph.2020.104672
- Pelepenko LE, Janini ACP, Gomes BPF, de-Jesus-Soares A, Marciano MA. Effects of Bismuth Exposure on the Human Kidney—A Systematic Review. Antibiotics (Basel). 2022;11(12):1741. Available from: https://doi.org/10.3390/antibiotics11121741
- Slikkerveer A, de Wolff FA. Pharmacokinetics and Toxicity of Bismuth Compounds. Medical Toxicology and Adverse Drug Experience. 1989;4:303-323. Available from: https://doi.org/10.1007/bf03259915
- Wang R, Li H, Sun H. Bismuth: Environmental Pollution and Health Effects. In: Encyclopedia of Environmental Health. 2019;415–423. Available from: https://doi.org/10.1016/B978-0-12-409548-9.11870-6
- Leussink BT, Slikkerveer A, Engelbrecht MR, van der Voet GB, Nouwen EJ, de Heer E, de Broe ME, de Wolff FA, Bruijn JA. Bismuth overdosing-induced reversible nephropathy in rats. Archives of Toxicology. 2001;74(12):745-754. Available from: https://doi.org/10.1007/s002040000190
- European Food Safety Authority. Food Contact Materials. 2024. Available from: https://www.efsa.europa.eu/en/science/scientific-committee-and-panels/fcm
- ECHA. 2024. Available from: https://chem.echa.europa.eu/100.028.343/dossier-view/1d8933e3-9db4-4860-822d-8c5a6b8e122e/IUC5-ef34b7bd-0070-4372-af9b-9f17b0900a79_49c29cca-df69-4dd2-a319-63142763597f?searchText=7440-69-9
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
