Author(s):
Denitza Zheleva*and Razvigor Darlenski
Department of Dermatology and Venereology, Tokuda Hospital Sofia, Bulgaria
Received: 10 February, 2017; Accepted: 23 February, 2017; Published: 24 February, 2017
Denitza Zheleva, Department of Dermatology and Venereology, Tokuda Hospital Sofia, 51B, Nikola Vaptzarov blvd, 1407 Sofia, Bulgaria, Tel: 00359 895 788551; E-mail:
Zheleva D, Darlenski R (2017) Atopic Eczema - From Epidemiology to Therapeutic Approach. Glob J Allergy 3(1): 004-010. DOI: 10.17352/2455-8141.000016
© 2017 Zheleva D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Atopic dermatitis is one of the epidemically expanding non-infectious diseases in the 21 century. It poses immense challenges to both patients and physicians. With a steady growth in its incidence and prevalence, the disease carries a heavy social and economical burden. Herein we discuss the therapeutic algorithm for atopic dermatitis in accordance with the disease severity. We emphasize on the personalized approach in selecting the proper treatment method and regimen for each patient.
Introduction
Atopic dermatitis (AD, syn. ‘atopic eczema’) is the commonest inflammatory skin disorder in children and represents a serious problem for the providers of health care all over the world [1-4] with an impressive effect on patients’ quality of life [1,5-9]. In 40–60% of paediatric patients with AD persist having symptoms later on in life [10,11]. Although AD often starts in early infancy, there are adult onset forms which start in adolescence or adulthood [11]. Most of the patients with AD can control their skin disease with topical therapy and emollient skin care. There is a considerable group of patients with severe AD who do not respond to the prescribed treatment with moisturizers, topical corticosteroids (TCS), and/or topical calcineurin inhibitors (TCI) or experience immediate flare-ups after tapering topical anti-inflammatory therapy.
The European Academy of Allergology and Clinical Immunology (EAACI) [12] suggested definition of the term atopy – ‘a personal or familial tendency to produce IgE antibodies in response to low doses of allergens, usually proteins, and to develop typical symptoms of asthma, rhinoconjunctivitis or eczema/dermatitis’. As an alternative term the authors also suggested ‘atopic eczema/dermatitis syndrome’ [12]. In 2003, the Nomenclature Review Committee of the World Allergy Organization (WAO) updated the EAACI 2001 position statement [13] and suggested that the term ‘eczema’ should replace the provisional term ‘atopic eczema/dermatitis syndrome’. The update further suggested that ‘eczema’ could be subclassified as ‘atopic eczema’ and ‘nonatopic eczema’ according to the presence or absence of IgE antibodies.
The pathophysiology and clinical phenotype of AD are very heterogeneous and change from patient to patient as well as within the history of a given patient. The disease has great impact over patients’ quality of life and beyond, a socio-economic burden. Moderate to severe AD causes over two billion euro in lost productivity in the European Union (EU) every year [14,15]. Lapidus et al., estimated that the annual amount spent on ambulatory care, emergency department care, inpatient care, and outpatient prescriptions for American children with AD is US$364 [16]. The cost of medical services and prescription drugs was estimated to be between US$0.9 and US$3.8 billion in the United States between 1997 and 1998.
Epidemiology
For the last 30 years there is a dramatic increase in the prevalence of AD. It has increased from 9% to 12% for 10 year period between 1960 and 1970 [17], and nowadays the prevalence is approximately between 15-20% [10,18]. In developed countries it is currently estimated between 10-20% for children and 1-3% for adults. Last studies reveal that the prevalence range in various borders according to the goegraphic regions and the environmental factors. It varies for different countries: less than 1- 2% in Iran and Albania, approximately 11 % for the United States, up to 16-17% in Japan and Nigeria. The Australasia and Northern Europe shows higher prevalences of the condition, while the prevalences reported in Eastern and Central Europe and Asia is lower [19]. AD often starts in early infancy; approximately 45% of all cases begin within the first 6 months of life, 60% during the first year, and 85% before 5 years of age. Up to 70% of these children outgrow the disorder before adolescence [20]. The prevalence of AD continues to increase, particularly in young children from low-income countries, such as in Africa and East Asia, where there is also often an urban–rural gradient of disease.
Therapeuthic approach
There is a wide variety of treatment strategies that has been established for AD. For most of the patients a combination of emollient and anti-inflammatory therapy is regarded as an optimal choice [21,22] (Table 1). A step-wise approach to AD treatment with regard to the disease severity is presented at Figure 1.
-
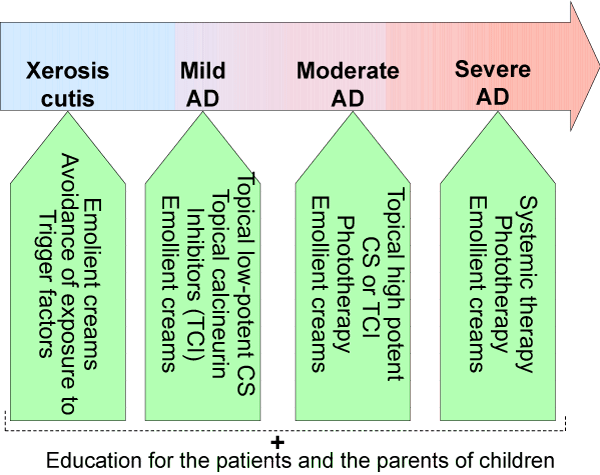
Figure 1:
Strategy in atopic dermatitis treatment according to the severity of the disease.
Non-drug therapy: emollients, bleach baths, wet wraps: The skin care aims to compensate the genetically determined impaired epidermal barrier function. The continous basic therapy includes emollient creams and emulsions even in periods and sites in which the AD is not active. There is a wide variety of emollients. The formulation of the emollient should be chosen based upon the degree of dryness of the skin, the sites of application, acceptance by the patient and the season. Ointments (e.g. petrolatum) and water-in-oil creams are more occlusive and tend to cause less burning and stinging than oil-in water creams and lotions. Potentially allergy-provoking ingredients such as perfumes, lanolin and herbal extracts should be avoided. The addition of moisterizing factors which are able to bind to the water (e.g. glycerol, urea) leads to increased hydration of the epidermis. However emollient products with higher concentration of urea or α and β- hydroxy acids, which can decrease scaling as well as dryness, tend to sting when used in children and on acutely inflamed and excoriated skin. Emollients containing paticular combinations of lipids that are normally present in the stratum corneum (e.g. cholesterol, fatty acids, ceramides) may optimize barrier repair. Emollients should be appalied twice to three times daily to the entire cutaneous surface. A lag time between topical drug and emollient application should be at least 30 to 60 minutes.
Another essential part of the basic skin care includes an appropriate bathing with mild non- alkaline cleansers. It’s not recommended to leave the children have long baths (greater than 10 to 15 minutes) and showers because they can dry out the skin. Adding bath oil could be beneficial for restoring the water-lipid mantle of the skin. There are two types of bath oils: emulsion and spreading ones. The emulsion oil mix in the water (dilute bleach baths) and they are usually taken for five to ten minutes twice per week. Shower gels containing oils are now emerging and would probably replace the classical bath oils.
For acute flares of AD, wet-wrap treatments are an important and powerful tool in managing the severe condition. Soaking and smearing involves soaking an affected body part or whole body bathing in plain water for 20 minutes to be followed immediately by smearing an ointment over the affected area, without drying the skin. Explanation and education is extremely important in the treatment of AD and WWT should only be employed by practitioners trained in its use. Specialized nursing care is essential, especially when using WWT for prolonged periods. A short course of 1 week treatment has a significant effect in improving patients’ itch, eczema, and overall well-being.
When dealing with infants and children it is important to involve actively the parents so they are partners in a reasonable overall treatment scheme. It is helpful to give them a written list of recommendations and to give them enough information. The parents are the most important member of the team, as they will settle on choices consistently with a major impact on their child’s current and future health. We have to make their problems together and to convince the parents that we are working together and as a team we will go through it.
Topical anti-inflamatory therapy
The approach to AD varies with the age of the patient and the type of manifestations. The classical approach is targeting acute flares with short- term treatment regimens, i.e. reactive management. On the other hand, a novel strategy is the so called proactive therapy. This is an alternative treatment that includes intensive topical anti- inflammatory therapy until all lesions have mostly cleared. After that the application of the anti-inflamatory agents is only twice weekly (on the affected areas) in combination with daily applications of emollients to unaffected ones (Figure 2). This modality is based on the recent insights into the underlying skin barrier defect and its relationship to subclinical inflammation in the clinically health skin of atopic patients [23].
-
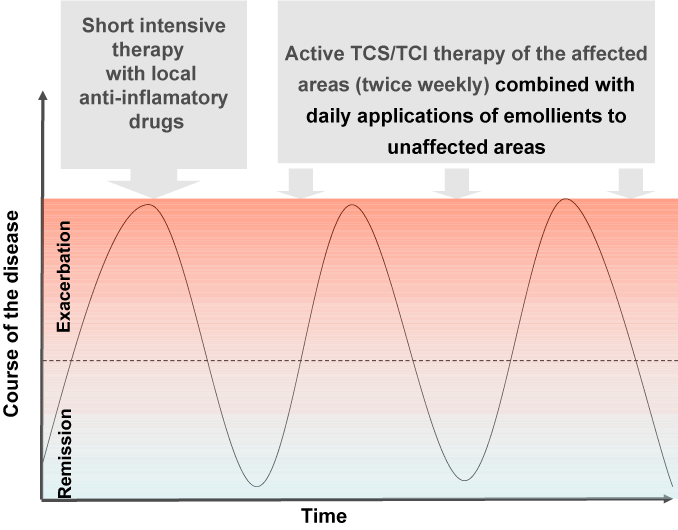
Figure 2:
Therapeutic regimen in proactive approach.
In the last 10 years proactive therapy was a major breakthrough for the topical anti inflamatory treatment of AD. It is evidence-based, immunologically founded treatment approach, based on the fact that normal-looking, nonlesional skin of patients with AD is not normal. Nonlesional AD skin looks normal to the naked eye, but harbours subclinical inflammation. Proactive therapy is defined as long-term, usually twice weekly, low-dose administration of anti-inflammatory therapy to previously affected skin, together with daily application of emollients to unaffected areas. In general proactive treatment with topical calcineurin inhibitors (TCI) and topical corticosteroids (TCS) leads to significant improvement in skin lesions, with high effectiveness for long-term maintenance treatment.
The mainstay of advanced treatment of severe AD in children includes TCS and TCI. Usually, the acute phases of AD are controlled with one to two applications per day of a potent TCS cream. Topical corticosteroids designed to have decreased systemic bioavailability and a favourable therapeutic index (e.g. prednicarbate, mometasone furoate, methylprednisolone aceponate, fluticasone, valerate and hydrocortisone butyrate) may be preferable especially for infants and young children with widespread involvement. The objective signs (erythema and oozing) and the subjective ones (itch and sleep loss) would be supressed within a few days, even so, tapering should not be initiated before the itch has disappeared. Dose tapering should be gradual to avoid withdrawal rebound; tapering strategies consist of using a less potent corticosteroid on a daily basis, or keeping a more potent one while reducing the frequency of application (intermittent regimen/ step-down (step-up) therapy in the maintenance phase).
Consideration in selecting the potency and vehicle of the topical corticosteroid include the location, type (e.g. acute versus chronic), thickness and extent of the AD lesions as well as the age of the patient. High-potency corticosteroids (particularly long-term use) should be avoided if possible for the face and body folds, due to risk of cutaneous atrophy. Potent corticosteroids are often needed for thick or lichenified plaques, nummular or prurigo-like lesions, and eczema on the palms and soles. Corticosteroid ointments (which minimize burning/stinging) and creams are generally preferred considering the dryness of the skin in AD patients and the emollient effect s of these vehicle.
TCI are reasonable option and there are two products that are approved for the treatment of AD - tacrolimus 0.03% and 0.1% ointment (for moderate to severe disease) and pimercolimus 1% cream (for mild to moderate disease). Both are approved for application in children older than 2 years although many clinicians prescribe these drug off-label in younger age groups. The major adverse effect of both medications is the burning at the site of application; they are not associated with cutaneous atrophy. TCIs are particularly suitable for AD affecting the face and intertriginous areas, and at sites where corticosteroid-induced skin atrophy is present. TCIs are also beneficial in patients with frequently flaring to persistent AD that would otherwise require almost continual use if topical corticosteroids.
A serios and frequent problem among parents of atopic children is the so called “topical cortico phobia”. More than 50% of the parents requested non-steroidal prescriptions due to concerns regarding skin atrophy and growth retardation [24]. Corticosteroid phobia frequently leads to the worsening of symptoms and prompts the consideration of alternative strategies. Recently, Moret et al. have developed a scale called TOPICOP to measure TC phobia among parents of children with atopic dermatitis [25].
Another important concern is about the association between TCI use and lymphoma risk in clinical practice, and the incidences of malignancy and lymphoma in clinical databases. To date, this risk remains theoretical and is based mainly on the drug’s mechanism of action, data from animal studies and a few single case reports of lymphoma and skin cancer in patients treated with TCIs (26). No definitive human clinical trial data has demonstrated an increased risk of malignancy with TCI exposure and that makes the validity of the boxed warning in large part questionable [27]. Even though the right therapeutical approch requires prescribing TCIs appropriately as corticosteroid-sparing agents and proactively.
Some new non-steroidal topical treatment has been approved for the treatment of patients with mild to moderate AD, recently [28]. Introducing topical phosphodiesterase inhibitors (crisaborole) in treatment of AD promises new horizons for patients with AD. According to the latest studies it is an alternative medication with favorable safety profile [29]. Crisaborole leads to reduction of the pruritus and showed significant improvement of the symptoms of the disease. Future studies will investigate potential risks in long term usage and patients younger than 2 years of age [30,31].
Phototherapy
Phototherapy is a second-line treatment, after failure of first-line treatment (TCS and TCI). UVA1, UVA combined with UVB and narrowband UVB have each been shown to improve both the eczema and associatied pruritus. Phototherapy can be used as maintenance therapy in patients with chronic disease. Phototherapy treatment of all forms should be under the guidance and ongoing supervision of a physician knowledgeable in phototherapy techniques. Psoralen plus UVA (PUVA) also brings a prompt improvement in severe atopic dermatitis. Bath PUVA therapy avoids the complications of systemic PUVA and appears well-suited for those patients. In addition, treatment with UVB has been shown to reduce S. aureus colonization of the skin in AD patients [32]. Phototherapy can be used as mono-therapy or in combination with emollients and TCS. The combined use of phototherapy with TCI is cautioned, as the manufacturers suggest limiting exposure to natural and artificial light sources while using these topical medications.
The light modality chosen should be guided by factors such as availability, cost, patient skin type, skin cancer history, and patient use of photosensitizing medications. The dosing and scheduling of light should be based on minimal erythema dose and/or Fitzpatrick skin type. Home phototherapy under the direction of a physician may be considered for patients who are unable to receive phototherapy in an office setting.
Systemic therapy
When optimized topical regimens and/or phototherapy do not adequately control the signs and symptoms of disease therapy with systemic immunomodulatory agents could be adimistered.
Only severe, refractory cases of AD should be treated systemically in case that they do not respond adequately to intensive topical therapy. Systemic cortocosteroid therapy have dramatic effect but they should be avoided due to the serious short- and long-term adverse effects and the serious rebound effect upon their discontinuation. When systemic corticosteroids are prescribed they should be used over 1-2 weeks and then tapered slowly.
The administration of systemic agents like Cyclosporine, Azathioprine, Methotrexate and Mycophenolate mofetil should be should be adjusted to the minimal effective dose once response is attained and sustained. Adjunctive therapies should be continued to use the lowest dose and duration of systemic agent possible. Treatment decisions should be based on each individual patient’s AD status (current and historical), comorbidities, and preferences.
In randomized controlled trials Cyclosporine (150-300 mg/daily; 3-6 mg/kg/daily paediatric) was approved as an effective and recommended as a treatment option for patients with AD refractory to conventional topical treatment. There are some serious side effects including nephrotoxicity (after 3-6 months of therapy) and increased blood pressure, which seem to be dose-dependent. Moreover cyclosporine is often not sufficient as mono-therapy, requiring combination with TCS to reach an almost complete remission.
Azathioprine (1-3 mg/kg/daily; 1-4 mg/kg/daily paediatric) is recommended as a systemic agent for the treatment of refractory AD. Once clearance or near-clearance is achieved and maintained, azathioprine should be tapered or discontinued, with maintenance of remission via emollients and topical agents. Concomitant phototherapy is not advised because of increased risk of DNA damage and possible photocarcinogenicity, particularly with UVA exposure. The activity of the enzyme thiopurine methyltransferase (TPMT) should be measured in pediatric patients at baseline, with repeated testing considered in cases of non-response or change in response. The dose should be adjusted according to the TPMT levels because of the risk of hepatotoxicity when the levels are higher. Similarly, children with lower TPMT levels may have improved clinical response on lower drug doses but may have an increased risk of myelosuppression.
Methotrexate (7,5-25mg/week; 0.2-0.7 mg/kg/week paediatric) has shown promising results in small series of adults with AD and had similar efficacy to azathioprine in recent randomized controlled trials [33]. Folate supplementation is recommended during treatment with methotrexate. Blood count and liver enzymes should be checked on a regular basis, i.e. every 2 weeks.
Another alternative variably effective therapy for refractory AD include mycophenolate mofetil (1.0-1.5 g orally twice daily; 1200 mg/m2 daily, which corresponds to30-50 mg/kg/daily paediatric). Successful long-term treatment for up to 29 months has been reported in 80% of patients [34,35]. In general mycophenolate mofetil is safe to use, nevertheless some side effects like nausea, vomiting, and abdominal cramping. Rarely, hematologic (anemia,leukopenia, thrombocytopenia) and genitourinary (urgency, frequency, dysuria) symptoms have been reported [36].
Several studies has shown reduction in both sypmtoms and signs of AD with Interferon gamma, which is is a dimerised, soluble cytokine whose action in AD is poorly understood. It has varying results but still only a limited role in the treatment of severe AD [36].
Targeted molecular therapy («Biologics»)
Recently it has been reported that there are more than 19 open studies testing systemic treatment in AD patients, many of which involve new biologic agents. The targeted molecular therapies include a wide variety of medications and they are currently under development for the treatment of asthma and AD. The anti-CD20 monoclonal antibody Rituximab has been shown in an open label trial to substaniatialy improve severe AD in adults. The anti- IgE monoclonal antibody Omalizumab is FDA- approved for the treatment of asthma in patients≥12 years old with sensizitation to aerollergens and a total IgE level up to 700 IU/ml.
Alternative and adjuvant treatment methods
Climate therapy: Climate therapy has significant role for the treatment of various chronic dermatological diseases as well as for pulmonary ones [37]. It combines anti-inflammatory treatment in a trigger-free environment with being hospitalized in a specialized clinic for a period of four weeks to three months. Climate therapy might be performed at the seaside or in mountain resorts (with mountin alltitude more than 2000m.) And it has shown improvement in disease activity and reduced corticosteroid use in patients with AD [38].
Textile materials: Avoiding some mechanic irritants like clothing with synthetic fabrics and wool textiles is crucial for the eczema stabilization. The selection of suitable textiles can reduce significantly the clinical severity of AD. There are recommendations for wearing non-occlusive, cotton garments for patients with AD [39]. On the other hand the high rate of cutaneous colonization with Staphylococcus aureus requires new therapeutic approach by using silver-coated textiles which have proved antibacterial effect. They are able to reduce the clinical severity of AD and S. aureus colonization significantly only in 2weeks [40]. There are two advantages of the silver products in the control of bacterial infections: a broad-spectrum antimicrobial activity and the absence of drug resistance [41]. S. aureus density remained significantly lower after stop clothing silver-coated textiles. Moreover they led to pronounced reduction of topical steroid use.
Complementary medicine (TCM): Nowadays complementary medicine has great impact on dermatologists’ strategies for solving different kind of skin problems. Complementary and alternative medicine (CAM) is able to prevent, cure, or relieve symptoms by using numerous diverse therapeutic concepts, ranging from as herbal medicine, diet with essential fatty acids, and probiotics, to acupuncture [42]. Oral administration of TCM capsule containg 5 types of herbs - Flos lonicerae, Herba menthae, Cortex moutan, Rhizoma atractylodis and Cortex phellodendri has antibiotic, anti-inflammatory and immunomodulating effects. Pharmacological studies have documented significant reductions in clinical severity of the disease and the pruritus [43]. There is also improvement in sleep and no apparent adverse effects [44]. The combination of acupuncture and Chinese herbal medicine have a beneficial effect on patients with atopic dermatitis and may offer better results than Chinese herbal medicine alone [45]. On the other hand the alternative therapy is not always helpful and still serious adverse effect are documentated. That explains the need of further randomized and placebo-controlled studies in the future.
Homeopathy: Homeopathy is currently gaining more and more popularity worldwide, especially in Europe, Latin America, and Asia. This controversial method is based on the principle of «similars», whereby highly diluted preparations of substances that can cause symptoms in healthy volunteers are used to stimulate healing in patients who have similar symptoms when ill [46]. Patients and physicians are quite intrested in this alternative method due to the lack of adverse effects and the good perspective for long term therapy. Diagnosis and prescriptions are made only by certified medical doctors with engough knowledge in this terapeuti approach. Several publicated studies confirm the effectiveness of the homeopathic intervention with significant improvement in the dermatological status [47]. Moreover the homeopathic intervention has led to modest reductionsin the use of medications commonly prescribed for AD [48].
Therpeutic patient education
Education is undoubtedly one of the most important factor for the adherence of the patients and their parents to the prescribed therapy. The question now is how to best deliver it, considering outcomes and cost. We can improve treatment adherence and lessen fears and misconsception by some educational methods .They might vary greatly in scope, intensity, frequency, setting, and personnel used. Disease-directed teaching can be on an individual or group basis. The so called eczema school has been established in some countries in formal, structured multidisciplinary educational programs [49]. An adjunction to the conventional therapy are hand outs with written instructions, video interventions, eczema workshops and nurse-led programs.
Conclusive Remarks
AD is a multi-factor disease that requests personalized approach. The step-wise model with regard to the disease severity provides a practical algorithm to the disease management. Patient and parent therapeutic education represent an important element of the treatment plan. The future of AD treatment belongs to novel biological therapeutic agents coming in the recent years.
-
- Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC (2005 )The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol 22: 192-199. Link: https://goo.gl/yOI8aE
- Kemp AS (2003) Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 21: 105-113. Link: https://goo.gl/wEqE6P
- Mancini AJ, Kaulback K, Chamlin SL (2008) The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol 25: 1-6. Link: https://goo.gl/sKBTMu
- Verboom P, Hakkaart-Van L, Sturkenboom M, De Zeeuw R, Menke H, et al. (2002) The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol 147: 716-724. Link: https://goo.gl/SYT7Uh
- Flohr C (2011) Recent perspectives on the global epidemiology of childhood eczema. Allergol Immunopathol (Madr) 39: 174-182. Link: https://goo.gl/3XTeZr
- Arnold RJ, Donnelly A, Altieri L, Wong KS, Sung J (2007) Assessment of outcomes and parental effect on Quality-of-Life endpoints in the management of atopic dermatitis. Manag Care Interface 20: 18-23. Link: https://goo.gl/MPoaxi
- Lewis-Jones S (2006) Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 60: 984-92. Link: https://goo.gl/tcbCDk
- Meltzer LJ, Moore M (2008) Sleep disruptions in parents of children and adolescents with chronic illnesses: prevalence, causes, and consequences. J Pediatr Psychol 33: 279-91. Link: https://goo.gl/anNSTo
- Weisshaar E, Diepgen TL, Bruckner T, Fartasch M, Kupfer J, et al. (2008) Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol 88: 234-239. Link: https://goo.gl/PT7TiM
- Wuthrich B (1999) Clinical aspects, epidemiology, and prognosis of atopic dermatitis. Ann Allergy Asthma Immunol 83: 464-470. Link: https://goo.gl/d0lhdU
- Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, et al. (2013) Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 68: 498-506. Link: https://goo.gl/4RvTRi
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, et al. (2001) A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 200156: 813-24. Link: https://goo.gl/j9rHrv
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, et al. (2004) Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 113: 832-836. Link: https://goo.gl/Ym31DC
- Zuberbier T, Orlow SJ, Paller AS, Taieb A, Allen R, et al. (2006) Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 118: 226-232. Link: https://goo.gl/ZlXsTc
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA (1996) The cost of atopic eczema. Br J Dermatol 135: 20-23. Link: https://goo.gl/6WBbzK
- Lapidus CS, Schwarz DF, Honig PJ (1993) Atopic dermatitis in children: who cares? Who pays? J Am Acad Dermatol 28: 699-703. Link: https://goo.gl/osXPS3
- Schultz Larsen F, Hanifin JM (1992) Secular change in the occurrence of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 176: 7-12. Link: https://goo.gl/1L8xGO
- Wuthrich B, Schmid-Grendelmeier P (2003) The atopic eczema/dermatitis syndrome. Epidemiology, natural course, and immunology of the IgE-associated ("extrinsic") and the nonallergic ("intrinsic") AEDS. J Investig Allergol Clin Immunol. 13: 1-5. Link: https://goo.gl/Abn107
- Williams H, Robertson C, Stewart A, Ait-Khaled N, Anabwani G, et al. (1999) Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol 103: 125-38. Link: https://goo.gl/6xA7J1
- Bieber T (2008) Atopic dermatitis. N Engl J Med 358: 1483-1494.
- Wollenberg A, Schnopp C (2010) Evolution of conventional therapy in atopic dermatitis. Immunol Allergy Clin North Am 30: 351-368. Link: https://goo.gl/mtEgu3
- Darsow U, Wollenberg A, Simon D, Taieb A, Werfel T, et al. (2013) Difficult to control atopic dermatitis. World Allergy Organ J 6: 6. Link: https://goo.gl/ntaziw
- Wollenberg A, Bieber T (2009) Proactive therapy of atopic dermatitis--an emerging concept. Allergy 64: 276-278. Link: https://goo.gl/Vum5T3
- Hon KL, Kam WY, Leung TF, Lam MC, Wong KY, et al. (2006) Steroid fears in children with eczema. Acta Paediatr 95: 1451-1455. Link: https://goo.gl/041xRw
- Moret L, Anthoine E, Aubert-Wastiaux H, Le Rhun A, Leux C, et al. (2013) TOPICOP(c): a new scale evaluating topical corticosteroid phobia among atopic dermatitis outpatients and their parents. PLoS One 8: e76493. Link: https://goo.gl/QZInF6
- Harcharik S, Emer J (2014) Steroid-sparing properties of emollients in dermatology. Skin Therapy Lett 19: 5-10. Link: https://goo.gl/IUBOix
- Siegfried EC, Jaworski JC, Hebert AA (2013) Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol 14: 163-178. Link: https://goo.gl/6pjhPt
- Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, et al. (2016) Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 75: 494-503 e4. Link: https://goo.gl/UoM4WJ
- Zane LT, Chanda S, Jarnagin K, Nelson DB, Spelman L, et al. (2016) Crisaborole and its potential role in treating atopic dermatitis: overview of early clinical studies. Immunotherapy 8: 853-866. Link: https://goo.gl/K9vDla
- Draelos ZD, Stein Gold LF, Murrell DF, Hughes MH, Zane LT (2016) Post Hoc Analyses of the Effect of Crisaborole Topical Ointment, 2% on Atopic Dermatitis: Associated Pruritus from Phase 1 and 2 Clinical Studies. J Drugs Dermatol 15: 172-176. Link: https://goo.gl/nxVXVB
- Jarnagin K, Chanda S, Coronado D, Ciaravino V, Zane LT, et al. (2016) Crisaborole Topical Ointment, 2%: A Nonsteroidal, Topical, Anti-Inflammatory Phosphodiesterase 4 Inhibitor in Clinical Development for the Treatment of Atopic Dermatitis. J Drugs Dermatol 15: 390-396. Link: https://goo.gl/mUl81J
- Dotterud LK, Wilsgaard T, Vorland LH, Falk ES (2008) The effect of UVB radiation on skin microbiota in patients with atopic dermatitis and healthy controls. Int J Circumpolar Health 6: 254-60. Link: https://goo.gl/r4zW8J
- Schram ME, Roekevisch E, Leeflang MM, Bos JD, Schmitt J, et al. (2011) A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol 128: 353-359. Link: https://goo.gl/evr9eU
- Grundmann-Kollmann M, Podda M, Ochsendorf F, Boehncke WH, Kaufmann R, et al. (2001) Mycophenolate mofetil is effective in the treatment of atopic dermatitis. Arch Dermatol 137: 870-873. Link: https://goo.gl/EyXx2X
- Murray ML, Cohen JB (2007) Mycophenolate mofetil therapy for moderate to severe atopic dermatitis. Clin Exp Dermatol 32: 23-27. Link: https://goo.gl/sgiryj
- Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, et al. (2014) Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 71: 327-349. Link: https://goo.gl/g6II0d
- Rijssenbeek-Nouwens LH, Bel EH (2011) High-altitude treatment: a therapeutic option for patients with severe, refractory asthma? Clin Exp Allergy 41: 775-782. Link: https://goo.gl/PZesFd
- Schuh A, Nowak D (2011) [Evidence-based acute and long-lasting effects of climatotherapy in moderate altitudes and on the seaside]. Dtsch Med Wochenschr 136: 135-139. Link: https://goo.gl/50BhTB
- Ring J, Brockow K, Abeck D (1996) The therapeutic concept of "patient management" in atopic eczema. Allergy 51: 206-215. Link: https://goo.gl/TwMhwt
- Gauger A, Mempel M, Schekatz A, Schafer T, Ring J, et al. (2003) Silver-coated textiles reduce Staphylococcus aureus colonization in patients with atopic eczema. Dermatology 207: 15-21. Link: https://goo.gl/qhFMmq
- Ricci G, Patrizi A, Bellini F, Medri M (2006) Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol 33: 127-143. Link: https://goo.gl/h2BiEY
- Boneberger S, Rupec RA, Ruzicka T (2010) Complementary therapy for atopic dermatitis and other allergic skin diseases: facts and controversies. Clin Dermatol 28: 57-61. Link: https://goo.gl/p4khES
- Hon KL, Leung TF, Wong Y, Lam WK, Guan DQ, et al. (2004) A pentaherbs capsule as a treatment option for atopic dermatitis in children: an open-labeled case series. Am J Chin Med 32: 941-950. Link: https://goo.gl/ApfsVG
- Hon KL, Lee VW, Leung TF, Lee KK, Chan AK, et al. (2006) Corticosteroids are not present in a traditional Chinese medicine formulation for atopic dermatitis in children. Ann Acad Med Singapore 35: 759-763. Link: https://goo.gl/5SAJQ7
- Salameh F, Perla D, Solomon M, Gamus D, Barzilai A, et al. (2008) The effectiveness of combined Chinese herbal medicine and acupuncture in the treatment of atopic dermatitis. J Altern Complement Med 14: 1043-1048. Link: https://goo.gl/yC3XqO
- Jonas W, Jacobs J (1996) Healing With Homeopathy. Link: https://goo.gl/7wjrKG
- Itamura R, Hosoya R (2003) Homeopathic treatment of Japanese patients with intractable atopic dermatitis. Homeopathy 92: 108-114. Link: https://goo.gl/9CFi6u
- Frenkel M, Hermoni D (2002) Effects of homeopathic intervention on medication consumption in atopic and allergic disorders. Altern Ther Health Med 8: 76-79. Link: https://goo.gl/7TxPbE
- Grillo M, Gassner L, Marshman G, Dunn S, Hudson P (2006) Pediatric atopic eczema: the impact of an educational intervention. Pediatr Dermatol 23: 428-436. Link: https://goo.gl/KaAAGR










Table 1:
Therapeuthic approach in atopic dermatitis.
Emollients
Bleach baths
Wet wraps
Topical corticosteroids
Topical calcineurin inhibitors
Topical phosphodiesterse inhibitors
UVA1, UVA combined with UVB and NB- UVB
Cyclosporine
Azathioprine
Methotrexate
Mycophenolate mofetil
Targeted molecular therapy ( "Biologics")
Climate therapy
Textile materials
Complementary medicine (TCM)
Homeopathy