Archives of Anatomy and Physiology
Ischemical Problems after Filler Injections: Anatomical Details and Practical Guide Lines to Avoid Disasters
Redaelli Alessio1*, Battistella Melania2 and Aleksey A Ivanov3
2Beauty House Spa, Ho Chi Minh City, Vietnam
3The Head of Department Topographic anatomy and operative surgery Pirogov Russian National Research Medical University, Moscow, Russia
Cite this as
Alessio R, Melania B, Ivanov AA (2017) Ischemical Problems after Filler Injections: Anatomical Details and Practical Guide Lines to Avoid Disasters. Arch Anat Physiol 2(1): 001-006. DOI: 10.17352/aap.000003Introduction: Soft-tissue filler injection is a very common procedure all around the world. Although the safety profile for these products is favorable, adverse events can occur, ranging from mild to severe in intensity. The most disastrous complication reported is accidental intra-arterial filler injection or in rare case due to the compression of the vessel associated with tissue injury and necrosis.
Objectives: The authors performed a literature search to identify the facial sites most prone to severe complications, especially due to anatomical structure. After a review of these complications they discuss preventive measures in order to avoid ischemical problems after filler injections and try to give a prompt intervention guidelines to manage this kind of complication and significantly decrease the risk of long term sequelae.
Method: The National Library of Medicine, the Cochrane Library, and Ovid MEDLINE were searched, and relevant articles were retrieved based on specific criteria. The complications reviewed included soft-tissue necrosis, filler embolization and visual impairment.
Results: Data collected from these case reports showed that the most common injection sites for necrosis are the glabella, the nose and the nasolabial fold. Blindness was most often associated with injection of the glabella. A thorough understanding of the facial vascular anatomy and a good injection technique reduce the risk of vascular occlusion.
Conclusions: Although soft-tissue fillers are a popular choice for minimally invasive rejuvenation of the face, physicians should be aware of the serious potential ischemical adverse event risks by using a correct technique of injection based on a good knowledge of anatomy and good instruments trying to prevent and/or manage its.
Introduction
The growing use of dermal fillers and its awareness among people can be explained by their effectiveness and versatility as well as from the always better quality and so of safety profile of the products used. The compatibility of hyaluronic acid with the human body and consequently its reversibility through using hyaluronidase enzyme make hyaluronic acid based dermal fillers the product of choice for many injectors.
Dermal fillers have been injected with increasing frequency over the last years for many aesthetic indications and especially for the treatment of aging face. The American Society of Plastic Surgeons (ASPS) in its last procedural statistic, reported that Soft tissue fillers raised 3% from 2013 to 2014 with 2.3 million procedures, second only to botulinum toxin type A and the trend is continuing to increase [1].
The continue widespread growing up use of dermal fillers coincide with a buildup of associated early and late complications of different severity level, even when the treatment is perfomed by the hands of an experienced injector. Most of the complications associated with hyaluronic acid filler use as edema, pain, erythema, itching and ecchymosis are normally mild, transient and reversible [2]. Serious complications are mostly related to vascular compromising and can result in the worst case in blindness [3] or cerebral ischemic events [4].
Ischemical damages can be provoke or by a direct occlusion after intravascular injection or because an interruption of vascular supply due to compression of the blood supply. In this specific case the compression on the vessel can be exercised by the volume of the filler itself on the vessel or by the enhancer material swelling [5]. All these elements can lead to a skin necrosis and can also leave scar [5].
Maximizing injection technique and thorough understanding of potential complications can help avoid, identify and manage them.
The sites commonly believed to be at greater risk for necrosis are glabella, nasal dorsum and nasolabial folds due to their critical structures and to their vascular supply [1,6,7].
The risk factors for vascular complications also include the volume injected and the site of application. In fact big volume injected at or near a vessel can lead to a major danger of arterial obstruction or indirect vessel compression. More attention should be taken when the injection has to be done in area with a scar tissue. Fibrotic tissue can stabilize arteries in a certain place making them easier to penetrate by a small sharp needles [8].
Most of the cases report immediate post-injection typical symptoms background presentation but there are a few ones in which symptoms appear only hours after procedure [9].
It can be considered an emergency and must be recognized and treated immediately [6], in order to minimize permanent damages.
The glabella is one the most common site of complications and many cases of necrosis after soft-tissue augmentation injections have been described. This event can happen because glabella is perfused by small vessels branching from the supratrochlear and supraorbital arteries and the collateral circulation is limited. The rare cases of visual impairment and blindness [4], after injection in this region can be possible because of the retrograde flow of intravascular injected material into the ophthalmic artery or into one of the distal branches that supply the retina and cornea. Cerebral ischemic events may also occur [3-10]. if the filler emboli reach the internal carotid artery and then propel into the intracranial circulation.
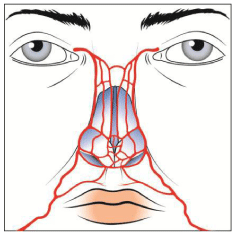
As regards the nasolabial fold and the dorsum of the nose are dangerous areas to inject because of their limited collateral circulation [11]. These area receive blood from angular artery and dorsal nasal artery [12] respectively and a wrong injection can lead to necrosis of the nasalala.
Variable baseline anatomy [13], is possible too and so has to be considered as well as all factors that can modify it. Prior facial surgeries or history of other soft tissue fillers injection are some of them. In case of these particular patient treatment, literature alert physicians also when the area to be injected is the one between the infraorbital and zygomatico-facial foramen [14].
Typical clinical signs of vascular impairtment include skin blanching followed by mottled discoloration called livedo reticularis. These symptoms are usually accompanied by a peculiar deep pain, out of proportion in terms of severity or persistence also after the treatment [15]. After digital compression, capillary refill is slow or completely absent with associated possible loss of function [8] and followed by dusky blue-red discoloration. A few days later blister formation and finally tissue slough are possible.
When the first symptom presents only several hours after it has been supposed that an embolus after the injection occurred and the poor collateral circulation results in a poor supply nutrition to the related area and in late necrosis [10]. In case of local anesthetic use, the clinical picture and symptoms can hide some symptoms of complications.
Anatomical study: dangerous areas
Since always anatomy has been essential for the better ubderstanding of physicians. In fact guided all the scientific community to find the best therapies trhough its knowledge. The dissection of cadaver has been fundamental in this process of evolution. Of course the respect for the cadaver. This paper shows some pictures made during a dissection for a deepest description of localization of the main arteries of the face that could be involved in the vascular sequelae post a filler injection.
Main arteries of the face
Temporal artery: It is one of the main arteries of the face. It divides in frontal and parietal artery. Then divides in many little arteries that provide blood supply for scalp and lateral face. Ischemical problems for injection of this artery after filler techniques may also occur. In the opinion of the authors the safest anatomic layer in temporal area is periosteum for needle’s technique with mandatory aspiration test [Figure 1].
Facial artery: Facial artery is the main artery for blood supply in anterior face. A long number of articles confirm its importance in ischemical side effects.
The facial artery arises from the point of connection of the anterior edge of masseter and the mandibular bone. Then it travels upwards to the medial orbital rim, where it anastomosis with orbital arteries (infraorbital a., supratochlear a.) [Figure 2].
Along its way until the nasal flare it is located under mimetic muscles in the deep fat layer. Two branches of facial artery (labialis superioris and labialis inferioris) are responsible for the blood supply of the perioral area. As a rule, labialis inferioris artery separates from the facial artery 1,5 cm above the mandibular edge and travels along the lower external edge of orbicularis oris. Labialis superioris artery separates from the facial artery at the level of the angle of the mouth and travels along the upper external edge of orbicularis oris. Often provides blood supply to the nasal flare (alar) [Figure 3].
Above the nasal flare the facial artery is located more superficially between mimetic muscle (orbicularis oculi and levator labii superioris alaeque nasi) or subcutaneously. The vessel is greatly reduced in diameter, and it can be easily damaged or compressed by fillers.
Although has been always a rare condition, the potential risk of vascular compromising or embolization and so skin necrosis in areas distant from the injection site after accidental intra-vascular injection of dermal fillers should be considered in case of injection into subcutis of the glabellar region, the nasal ala and nasolabial folds [Figure 4].
The physiopathologic mechanism is still not well known but it has been considered that the interruption of the vascular supply to the area due to direct injury to the vasculature and arterial obstruction by fillers secondary to its hydrophilic action. An excessive amount of dermal fillers can cause vascular compression resulting into a reduction in skin perfusion. Crust formation is initiated over the erythematous base along with a rim of fibrous tissue. Due to the replacement of normal tissues by fibrous material, the healing process may result in scar formation in spite of debridement and aggressive dressing changes.
These dangerous zones are vulnerable to tissue necrosis because of a main reason: a particular region strictly dependent on a single arterial branch and the interconnections between specific arteries. In the opinion of the authors quit all the main necrosis complications are due to ischemical problems much more then compressive problems.
The compression of the facial artery from nasolabial folds injection or compression of the columnellar artery are quite impossible to be reached and this is why after introduction of injections with cannula nasal tip or alar necrosis are reduced to a very low rate [17].
The compression of suprathroclear, supraorbital or glabellar arteries can lead to glabellar necrosis, but also in this case in the opinion of the authors quit all the cases of necrosis are due to an intraarterial injection [18].
This is why luckily a good injection technique and the right plane of injection can preserve physicians from this risk [13] [Figure 5].
Guidelines
The authors reviewed the available literature in order to better elucidate a standardized approach to the use of hyaluronidase to treat ischemical complications after hyaluronic acid filler injection [19]. National Library of Medicine’s PubMed database was utilized to have an overview of the efficacy, safety and outcomes and to finally find out the optimal use of hyaluronidase.
Even if there is not yet a standardized treatment modality for the correction of hyaluronic acid-filler injections, the authors suggest the following in order to minimize the risk of vascular complications:
∗ Understanding of the facial anatomy and influence of facial aging on the structures beneath the skin
∗ Identify the right anatomical plane that is different for any area and indications [20].
∗ Aspiration before each injection to be assured that the tip is not within a vessel [21].
∗ Use low pressure injections and inject minimal volumes and keeping the needle moving. It is suggested to use bolus injections only in the periosteum plane
∗ If patients feel pain during a bolus technique, the injection must be immediately stopped
∗ If a bleaching is visible during all techniques, the injection must be immediately stopped
∗ Avoid using anesthesia with adrenalin or epinephrine that may induce vascular spasm and hide signs of pain and blanching [9].
∗ Avoid injections in areas of previous scarring and if injecting these areas inject very slowly with prudence
∗ Use a blunt tip micro-cannula with bigger size 22 G or 25 G, which reduces the risks of intravascular injection of filler [8,22,23] and of disrupting key structures
∗ Use hyaluronic acid based product that is a temporary and biodegradable product and can be in part break down by hyaluronidase [9-24].
This enzyme acts by hydrolyzing hyaluronic acid by splitting the glucosaminidic bond between C1 of the glucosamine moiety and C4 of the glucuronic acid [25]. Several formulations of hyaluronidase like amphadase (derived from bovine testicular hyaluronidase), vitrase (derived from ovine hyaluronidase) and hylenex (a recombinant human hyaluronidase) are present in the market. When is possible, preliminary skin testing is recommended for vitrase and amphadase because of their animal origin [26]. In Italy a galenic Hyaluyronidase is available already diluted, 300UI in 3 ml.
In the event of vascular complication, based om the literature it is suggested to start and treat at the first sign following these simple recommendations in order to minimize the risk of necrosis and scar:
∗ Stop the injection immediately
∗ Massage the area for some minutes to try dissolve the bolus (8). There are few cases reported in which only with this shrewdness doctors solved the cases
∗ If the massage is not enough apply hot/warm water gauze and topical 2% nitroglycerin paste to stimulate quick vasodilation and restore blood supply [27], to the affected area as frequently as every 1 to 2 hours initially and disburse the bulk of the filler material respectively [28].
∗ If also this is not effective and in the case of hyaluronic acid use, the first step and gold standard is an immediate hyaluronidase injection to try to avoid tissue necrosis [28]. Hyaluronidase is sold in vials 1500IU (to be storage in the fridge at2-8 degrees). We dilute this vial with 1 ml normal saline solution. With a syringe of 1 ml we take 0,1(150 IU) and we diluite again up to 1 ml and so we obtain 15 IU in 0,1 ml. Normally boluses of 15 IU each up to 100, 200 IU are immediately injected in the ischemic area to try and dissolve the hyaruronic acid. It may be injected directly into the affected site, with doses of 40U or more per cm2, and starts to dissolve the material immediately and lasts for between 24 and 48 hours [8-29].
∗ Hyaluronidase can be diluted also with lidocaine to induce vasodilatation [30]. An extra volume of hyaluronidase can be injected (up to 4 cycles) if there is no clinical improvement after 60 min [31]
∗ If ophthalmic artery necrosis is suspected, the authors recommend immediately contacting oculoplastic surgeon for urgent retrobulbar injection of hyaluronidase which can dissolve intravascular as well as extravascular hyaluronic acid [32]
∗ To prevent the further clot formation it is recommended to start treatment with 2 pills of 325 mg of aspirin daily for a week in association with gastroprotective (as pantoprazol 20 mg) to prevent gastritis [33]
∗ In severe cases, low-molecular-weight heparin and systemic anticoagulation may be helpful. Prostaglandin E1 (PGE1) can also be used to promote vasodilation. When potent vasoactive drugs are used, the patient shoul be carefully monitored in the hospital [15]. Even if patient presentation is delayed, treatment is still recommended because it may restore normal circulation and speed the healing process [34-35]
∗ Oral prednisone, 20 to 40mg daily, for three to five days is recommended because it helps to decrease the inflammatory component of the injury and prevent further vascular compromise [27-28]
∗ If necrosis is progressive and not responsive to the above treatments, hyperbaric oxygen therapy should be considered [36]
∗ Of note, topical oxygen therapy, low molecular weight heparin, systemic steroids, sildenafil, platelet-rich plasma, filler removal through puncture and intravenous prostaglandin are reported to be beneficial [15-35-37-38]
∗ Localized skin breakdown should be treated with topical (with or without systemic) antibiotics, and antivirals should be used especially if necrosis is around the mouth [8-9]
∗ Also adipose-derived stem cell injection to boost the healing process and tissue regeneration promoting angiogenic processes. However, exact mechanisms are not yet come out into the open [39]
∗ Keeping the wound covered with ointment to prevent crusting and keeping out bacteria until healing is complete is important.
Eventual resulting scar can be improved with silicone pads, intralesional steroid injection, light dermabrasion surgical revision, CO2 laser or injection with filler to restore the contour.
The adverse events of hyaluronidase are uncommon. The ones more reported are directly related to the injections. Less than 0.1% of patients injected with hyaluronidase develop urticaria and angioedema. More caution has to be taken in patients with a history of bee allergy because hyaluronidase is considered to be one of the active components in bee venom [27-40]. There are no reported anaphylactic reactions after subepidermal injections [29-44].
Discussion
With increased popularity of hyaluronic acid fillers, hyaluronidase had become an indispensable tool in aesthetic medical clinics. It can be considered a safe and reliable tool for treatment of hyaluronic acid-induced complications. If the complication occurs with a hyaluronic acid filler, hyaluronidase is recommended. Hyaluronidase works to break down and hydrolyze hyaluronic acid. Hyaluronidase has been shown to have edema-reducing benefits and theoretically reduces occluded vessel pressure. Some authors have experience canalizing an occluded vessel and injecting hyaluronidase with immediate reversal of signs of occlusion.
The occlusion of vessels becomes quit always for an intra-arterial injection. To avoid this problem the aspiration test is always mandatory. Some authors say that the aspiration test is not useful and that it is impossible aspirate blood with fillers syringes. Well, in the last year it is direct experience of the authors have an aspiration test positive as seen in Fig. 6. For this reason the Authors strongly recommend the aspiration test in all injections of boluses of Hyaluronic acid in the same point. Cannulas on the contrary do not need aspiration test being it a retrograde technique [Figure 6].
Conclusion
Ischemical complications can be a very rare but potentially devasting problem sometimes of difficult solution. Deep anatomical knowledge and well assessed technical details are mandatory to reduce this risk to a very low percentage and to assure all patients to get the best possible treatment of the complication. In general the correction of nose, nasolabial folds and glabellar wrinkles with hyaluronic acid fillers can lead to impending nasal and/or glabellar skin necrosis, quit always caused by intravascular occlusion and possible embolism. The key for preventing the skin ischemia from progressing to necrosis is to identify and treat the ischemia as early as possible. Several symptoms and signs that includ intense pain immediately after the injection and discoloration of the skin, should be identify like a red flag. Any patients suspected to have a vascular experience should be Immediate or earliest as possible (<2 days) managed with a combination of treatments. Hyaluronidase has a primary role. In addition to this the underlying facial anatomy, good product and legal procedures are imperative to delivering the best patient care.
- https://www.plasticsurgery.org/news/press-releases/plastic-surgery-statistics-show-new-consumer-trends Link: https://goo.gl/Prx8cU
- Philippe L, Anthony B (2010) Fillers: contraindications, side effects and precautions. J Cutan Aesthet Surg 3: 16-19. Link: https://goo.gl/f8ioD8
- Carle MV, Roe RH, Novack RL (2015) Occlusion caused by cosmetic facial filler injection – reply. JAMA Ophthalmol 133: 225. Link: https://goo.gl/7i4Skp
- DeLorenzi C (2014) Complications of injectable fillers, part 2: vascular complications. Aesthet. Surg J 34: 584-600. Link: https://goo.gl/IXjNAx
- Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, et al. (2013) Complications following injection of soft-tissue fillers. Aesthet Surg J 33: 862-877. Link: https://goo.gl/yAg63b
- Brennan C (2014) Avoiding the “danger zones” when injecting dermal fillers and volume enhancers. Plast Surg Nurs. 34: 108-111. Link: https://goo.gl/ODCEhc
- Redaelli A (2011) Facial aging: medical, surgical and odontostomatological solutions. OEO Firenze Link: https://goo.gl/GLLzjc
- DeLorenzi C (2014) Complications of injectable fillers, part 2: vascular complications. Aesthetic Surg J 34: 584-600 Link https://goo.gl/RJBzWL
- Bruna SFB, Laila KDAB, Camila RMDR, Carolina BDSP, Carolina MT, Roberta TDS, et al. (2015) Delayed-type Necrosis after Soft-tissue Augmentation with Hyaluronic Acid. J Clin Aesthet Dermatol 8: 42-47. Link: https://goo.gl/GHwrMi
- Kim SN, Byun DS, Park JH, Han SW, Baik JS, et al. (2014) Panophthalmoplegia and vision loss after cosmetic nasal dorsum injection. J Clin Neurosci 21: 678-680. Link: https://goo.gl/WMvy26
- Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, et al. (2011) Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg 64: 1590-1595. Link: https://goo.gl/DF63j0
- Redaelli A (2015) Medical rhinoplasty: basic principles and clinical practice. 2nd revised edition. OEO Firenze 2015 Link: https://goo.gl/Xvdcc6
- Tansatit T, Apinuntrum P, Phetudom T (2016) Facing the Worst Risk: Confronting the Dorsal Nasal Artery, Implication for Non-surgical Procedures of Nasal Augmentation. Aesthetic Plast Surg. Link: https://goo.gl/hPA8ks
- Li X, Du L, Lu Jian-jian L (2015) Novel Hypothesis of Visual Loss Secondary to Cossmetic Facial Filler Injection. Ann Plast Surg 75: 258-260. Link: https://goo.gl/MClpg8
- 13 Park TH, Seo SW, Kim JK, Chang CH. Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr Aesthet Surg 64: 892-896 Link: https://goo.gl/8lTETk
- Funt D, Pavicic T (2013) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 6: 295–316. Link: https://goo.gl/1WxBai
- Levy LL, Emer JJ (2012) Complications of minimally invasive cosmetic procedures: prevention and management. J Cutan Aesthet Surg 5: 121-132. Link: https://goo.gl/1CFMpO
- Hong JY, Seok J, Ahn GR, Jang YJ, Li K, et al. (2016) Impending skin necrosis after dermal filler injection: A "golden time" for first-aid intervention. Dermatol Ther. Link: https://goo.gl/vvFD8G
- Van Loghem JA, Humzah D, Kerscher M (2016) Cannula Versus Sharp Needle for Placement of Soft Tissue Fillers: An Observational Cadaver Study. Aesthet Surg J. Link: https://goo.gl/9SF9k9
- Chen Q, Liu Y, Fan D (2016) Serious Vascular Complications after Nonsurgical Rhinoplasty: A Case Report. Plast Reconstr Surg Glob Open 4: e683. Link: https://goo.gl/JRNZ9E
- Buhren BA, Schrumpf H, Hoff NP, Bölke E, Hilton S, et al. (2016) Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur J Med Res 13: 21. Link: https://goo.gl/D8CFwo
- Jahn K, Homey B, Gerber PA (2014) Management of complications after aesthetic hyaluronic acid injections. Hautarzt 65: 851-853. Link: https://goo.gl/6cFGMd
- Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, et al. (2012) Blindness following cosmetic injections of the face. Plast Reconstr Surg 129: 995-1012. Link: https://goo.gl/CvkEQR
- Sánchez-Carpintero I, Candelas D, Ruiz-Rodriguez R (2010) Dermal fillers: types, indications, and complications. Actas Dermosifiliogr 101: 381-393. Link: https://goo.gl/XrPcU1
- Rebecca Small, Dalano Hoang (2012) A practical guide to dermal filler procedure. Ed. Lippincott Williams & Wilkins. Link: https://goo.gl/8zkBaO
- Funt D, Pavicic T (2013) Dermal fillers in aesthetics: an overview of adverse events and treatment approaches Clin Cosmet Invest Dermatol 6: 295-316. Link: https://goo.gl/S2gfZC
- Sánchez-Carpintero I, Candelas D, Ruiz-Rodriguez R (2010) Dermal fillers: types, indications, and complications. Actas Dermosifiliogr 101: 381-393. Link: https://goo.gl/XrPcU1
- Kassir R, Kolluru A, Kassir M (2011) Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature J Cosmet Dermatol 10: 224-231. Link: https://goo.gl/Vi9i0A
- Gilbert E, Hui A, Meehan S, Waldorf HA (2012) The basic science of dermal fillers: past and present Part II: adverse effects J Drugs Dermatol 11: 1069-1077. Link: https://goo.gl/kKpwkb
- Dayan SH, Arkins JP, Mathison CC (2011) Mathison Management of impending necrosis associated with soft tissue filler injections J Drugs Dermatol 10: 1007-1012. Link: https://goo.gl/EOmvme
- K. Beer, J. Downie, J. Beer (2012) A treatment protocol for vascular occlusion from particulate soft tissue augmentation. J. Clin. Aesthet. Dermatol. 5: 44–47. Link: https://goo.gl/OZmmvB
- Lee A, Grummer SE, Kriegel D, Marmur E (2010) Hyaluronidase. Dermatol Surg 36 :1071–1077. Link: https://goo.gl/RQvPsS
- Cohen JL, Biesman BS, Dayan SH, Claudio DeLorenzi, Lambros S, et al. (2015) Treatment of Hyaluronic Acid Filler-Induced Impending Necrosis With Hyaluronidase: Consensus Recommendations. Aesthet Surg J 35: 844-849. Link: https://goo.gl/oHFISR
- J.L. Cohen, B.S. Biesman, S.H. Dayan, Claudio DeLorenzi, Lambros S, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations Aesthet. Surg. J. (2015) Link: https://goo.gl/cqP5Q6
- J.D. Carruthers, S. Fagien, R.J. Rohrich, S. Weinkle, A. Carruthers (2014) Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast. Reconstr. Surg 134: 1197–1201 Link: https://goo.gl/lihTL2
- Dayan SH, Arkins JP, Mathison CC (2011) Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011 Sep;10(9):1007-12 Link: https://goo.gl/wPAfkB
- Kleydman K, Cohen JL, Marmur E (2012) Nitroglycerin: a review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol Surg 38: 1889–1897. Link: https://goo.gl/7TR1sx
- Beleznay K, Humphrey S, Carruthers JD, Carruthers A (2014) Vascular compromise from soft tissue augmentation: experience with 12 cases and recommendations for optimal outcomes. J Clin Aesthet Dermatol 7: 37–43. Link: https://goo.gl/3muOSH
- S.H. Dayan (2013) Complications from toxins and fillers in the dermatology clinic: recognition, prevention, and treatment. Facial Plast Surg. Clin. North Am 21: 663–673 Link: https://goo.gl/8PA9LI
- Daines SM, Williams EF (2013) Complications associated with injectable soft-tissue fillers: a 5-year retrospective review JAMA Facial Plast Surg 15: 226–231. Link: https://goo.gl/vbhWDY
- B.K. Kang, I.J. Kang, K.H. Jeong, M.K. Shin MK (2015) Treatment of glabella skin necrosis following injection of Hyaluronic acid-filler using platelet-rich plasma. J. Cosmet. Laser Ther 18: 111-112. Link: https://goo.gl/DM6HWZ
- Sung HM, Suh IS, Lee HB, Tak KS, Moon KM, et al. (2012) Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 39: 51–54. Link: https://goo.gl/yefIPZ
- Muller UR (2011) Hymenoptera venom proteins and peptides for diagnosis and treatment of ven-om allergic patients. Inftamm Allergy Drug Targets.10: 420–428. Link: https://goo.gl/IFdO7V
- Balassiano LKA, Bravo BSF (2014) Hyaluronidase: a necessity for any dermatologist applying injectable hyaluronic acid. Surg Cosmet Dermatol. 6: 338–343.. Link: https://goo.gl/tGvIXa
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.






 Save to Mendeley
Save to Mendeley
