Archives of Anatomy and Physiology
Nitric oxide functions in the heart
Tarik Kivrak1*, Kenan Erdem2 and Ilgin Karaca2
2Department of Cardiology, Medeva Hospital, Konya, Turkey
Cite this as
Kivrak T, Erdem K, Karaca I (2017) Nitric oxide functions in the heart. Arch Anat Physiol 2(1): 020-026. DOI: 10.17352/aap.000007Nitric oxide (NO) is an important organizer of the cardiovascular function and is an important mechanism in hampering the pathogenesis of the diseased heart. The scenario of bioavailable NO in the myocardium is complicated: 1) NO obtain from both endogenous and exogenous NO synthases (NOSs) and the number of NO from exogenous sources varies considerably. 2) NOSs are located at separated regions of cardiac cells and are organized by varied ways under stress.3) NO arranges various target proteins via different ways of post-transcriptional modification which are soluble guanylate cyclase [sGC]/cyclic guanosine monophosphate [cGMP]/protein kinase G [PKG]-dependent phosphorylation, S-nitrosylation, and trans-nitrosylation. 4) the downgradient stabilizers of NO differ from proteins and enzymes in the mitochondria and membrane.5) NOS generates several radicals in addition to that NO (varied NO-associated yields) and stimulates redox responses. But, NOS inhibits cardiac oxidases to diminish the sources of oxidative stress in diseased hearts. Recent consensus indicates the importance of nNOS protein in cardiac protection under pathological stress and NO-dependent mechanisms are better understood in healthy and diseased hearts.
Introduction
Nitric oxide (NO) is a primary matter that plays important roles in maintaining cardiovascular functions in humans [1–5]. The NO that argues positions in the myocardium can be obtained via exogenous matters or is generated from the endogenous NO synthases and alertable NOS by inflammatory cytokines following infection [4–6]. The impacts of NO on myocardial functions and the roles for NOS in diseased hearts has improved in the last times. Overall approaches have been assumed to succeed this outcome, containing manipulation of stabilizers of to supplementation of NO mimetics, and precise detection of plasma and tissue NO [4,5,7,8]. Even so, the practical arguments of NO and its regulators in therapeutic strategies in cardiovascular diseases hamper because of the compound nature of NO and the array of downstream signaling cascades and stabilizations in the myocardium.
Resource of NO and effect mechanisms
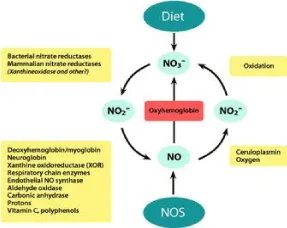
It is affirmed that NO is varied from the standard L-arginine–NOS–NO way. Actually, NO that performs functions in the myocardium may also be obtained from the other source of NO. Nitrate (NO3 −) in many types of green vegetables [9–11], is taken up into the plasma to become a safe reservoir and the decisive pioneer of NO. Nitrate from this source is taken on by the salivary , is secreted in the intensified form in the saliva, and is right after diminished to more active nitrite (NO2 −) in the oral cavity by nitrate reductases of commensal bacteria. Nitrite is decreased to NO in the stomach and is sucked up into the plasma in the gastrointestinal system. Many proteins are known to be contained in the NO metabolite cycle and nitrite’s reduction to NO, including xanthine oxidase [12,13], deoxyhemoglobin and deoxymyoglobin [14–16], neuroglobin [17], respiratory chain enzymes [18], cytochrome P450 [19], aldehyde oxidase [20], carbonic anhydrase (21) and NO synthase [22,23].Indepth, up to 25% of nitrate re-uptake by the salivary and generates NO in the circulation; the rest of the nitrate is secreted in the urine. The number of NO from exogenous can be as high as the number that is generated from NOS in the tissues, indicating the importance of this pathway in supplementing local NO in the tissue. The effectiveness, food derived functional NO is oxygen independent [10,11]. Accordingly, NO from this source becomes more important in ischemic conditions, like myocardial infarction. NO can undergo an oxidative process via nNOS and generate decisive nitrate, which can be diminished back to nitrite and NO by nitrate reductases, such as xanthine oxidoreductase or aldehyde oxidase [10,11]. Consequently, there is a fixed of NO metabolites, and NO that maintains exogenous NO in the body. The respective additives of the endogenous versus exogenous NO to intracellular signaling and function in hearts in vivo remain to reveal. Some tissues are the active sites for NO production from constitutive NOS. Lately, it has demonstrated that muscle is a nitrate store up that gains plasma NO because of the wideness of the tissue in the body [24]. nNOS in the skeletal muscle promotes to the supply because it is the isoform in the skeletal muscle [25]. But, the NO from the specific sources that promote to the bioavailable NO in the myocardium. eNOS is the main isoform of NOS that plays significant roles in NO regulation of functions in the majority of tissues, including the heart [4,5,7,8]. In the myocyte, eNOS is located in the plasma membrane, golgi apparatus, nucleus, and mitochondria [8,26]. eNOS exhibits the highest activity at the membrane, followed by outer layers of the cis-Golgi and little activity in the cytosol, nucleus, and mitochondria [26,27]; therefore, localization is the primary determinant of eNOS activity for specific biological functions. Conversely, mislocalization of eNOS has been demonstrated to diminish its capacity to produce NO in cells [26–28]. The last consensus is that nNOS is the isoform that plays the major role in cardiac tissue because nNOS is showed in all heart [7,8]. As such, nNOS is well placed to fill primary roles in modifying sympathetic and parasympathetic tones.nNOS is localized in the sarcoplasmic reticulum (SR) [6], and is contained in the Ca2+ handling processes of cardiac excitation-contraction coupling in the myocardium [7,8]. Lately, A study has demonstrated that nNOS is upregulated in the myocardium from the disease progression [29-32], and facilitates lusitropy through myofilament Ca2+ desensitization [32,33]. Until now, most of the responses of nNOS attributed nNOSα or nNOSμ [7,8]. But, the existence of many splice variants of nNOS (nNOSβ, nNOSγ, and nNOS2) suggests that lie variants of nNOS may be contained in generating NO and organizing a contractile function in the heart. Recently, we have presented new evidence to demonstrate that nNOSβ, which does not have the PDZ domain, is stated in the cardiac myocytes from the hearts of rats [34]. These results indicate that nNOSβ may play the major roles in myofilament. It has been reported in skeletal muscle that nNOSβ is stated in the Golgi apparatus and mediates myofilament regulation during exercise [25]. An overall understanding of nNOS and its tie various in the organelles and their roles in cardiac function in the hearts. Remarkable, the co-existence of eNOS and, nNOS and their many ties in the myocardium [7,8,35]. A conflictive leaning presents this in protein activities of eNOS and nNOS in the frailty heart; namely, eNOS expression is diminished considerably, while nNOS activity is enhanced [30–32,36]. Otherwise, nNOS to protect the myocardium from Ca2+ oxidative stress [7,31,37], and both eNOS and nNOS influence intracellular Ca2+ in the cells, and eNOS enhanced Ca2+ transients in myocytes in response to increased preload [38]. Contrary, nNOS induced spontaneous Ca2+ [39]. Spatial redistribution of NOSs is related to both the changes of activity and the removal of the primary targets. Virtually, the nNOS may be useful in protection its business to apply for myocardial protection.
NO synthase
Many mechanisms correlator the impacts of nitric oxide synthase. It approves that soluble guanylate cyclase (sGC)/cyclic guanosine monophosphate (cGMP) are the primary mechanisms that intercede the effects of NO in the body. The previous mechanism contains in post-translational modification of thiol in proteins by NO [40,41]. Protein-protein transfer of NO is known to present the most important mechanisms of NO [42]. In this process, the SNO proteins are referred to as nitrosyl asses. Trans- S-nitrosylation possesses advantages for efficient interactions between proteins [43].Furthermore, trans-nitrosylation is important when NO bioavailability is limited in an oxidative and nitrosative stress environment, such as during ischemia reperfusion. S-nitrosylation can be ceased by the action of nitrosylates, with NADH and NADPH serving as electron donors to regenerate glutathione and thioredoxin [44,45]. Many types of proteins are targeted by NO e.g. inhibition of protein phosphatase 2A/ protein phosphatase one by NO causes protein kinase A (PKA) and phospholamban (PLN) [46], while sGC activation by NO in the myocardium of rats [32]. Contrary, phosphodiesterase 5 (PDE5) reactivation by NO system limits cytosolic cGMP, a negative feedback mechanism of NO regulation of cGMP in cardiac myocytes [47]. Additionally, by targeting cardiac oxidases, such as xanthine oxidoreductase [48], NADPH oxidase [49,50], and mitochondrial reactive oxygen species (ROS) production [51], nNOS-derived from NO controls in the myocardium. Cysteine residues are the targets of ROS to cause S-glutathionylation in the proteins [52,53]; thus, S-nitrosylation by NO may block cysteine residues from irreversible oxidation under the conditions. Eventually, post-transcriptional terms downstream of NO change the proteins, altering their activity, and function, as well as, nNOS has been showed to generate H2O2 in the endothelium of arteries, for example, the aorta, and H2O2 mediates endothelium-dependent vascular relaxation (54,55). Contrary, blight of endothelial has been demonstrated to worsen endothelial function in some diseases [56-58], likewise, both eNOS and nNOS promote to acetylcholine stimulation of vasodilatation [55], by regulating protein kinases and phosphatases [59–61]. Conversely, uncoupling of eNOS and nNOS [48,62–64], results in the production of superoxide (O2 −) in return for NO; eNOS and nNOS occur the oxidative stress for pathological progression in the heart. nNOS performs its cardiac protection via the ion channels, modulating abnormal Ca2+ homeostasis, and mitochondrial function for the pathological process [7,8]. nNOS organizes ion channels and Ca2+-handling proteins.Specially, nNOS has permanently been demonstrated to diminish Ca2+ influx via the L-type Ca2+ channel (LTCC) [65]. In support of this, nNOS enhances the vulnerability of the LTCC for Ca2+-dependent inactivation in hypertension [66] where intracellular Ca2+ transient is increased secondary to nNOS-dependent myofilament Ca2+ desensitization (34). Variation of the LTCC by nNOS may prohibit extreme intracellular Ca2+ in myocytes under pathological situations. The ryanodine receptor (RyR) by nNOS has been contained in diminishing diastolic Ca2+ leak [67], increasing RyR open probability, and growing contraction in cardiac myocytes [74].Thus, nNOS protects against arrhythmogenesis by modulating Ca2+ transients [68-70].Besides, nNOS activity at the plasma membrane causes more significant Na+ influx via voltage-gated sodium channels via S-nitrosylation and increases the susceptibility of the myocardium for long QT and arrhythmias (34). Potassium channels are also potential targets of nNOS through S-nitrosylation and/or cGMP/PKG-dependent phosphorylation [71–73], which may play significant roles in the formation of cardiac function in hearts. NNOS-derived NO can cause S-nitrosylation of the SR calcium ATPase (SERCA) both under basal conditions [70,74]. Inhibition of nNOS decreases S-nitrosylation of SERCA at baseline level, and this is related to reduced Ca2+ uptake in the SR and decreased relaxation [74]. But, the functional important of this formation under disease situations survives to be detected. These results consider that the modes of post-transcriptional modification that underlie the specific impacts of nNOS are excessively dynamic, and this may optimize its formation of the target proteins under many stimuli, containing pressure overload.
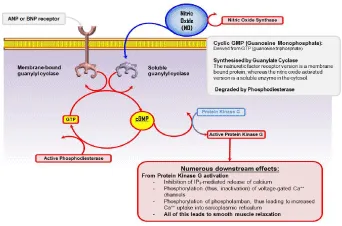
A recent study has demonstrated that nNOS enhances cGMP/PKG-dependent phosphorylation of cardiac troponin I and cardiac myosin binding protein C and contributes myocyte relaxation in hypertension via cGMP/PKG-dependent myofilament Ca2+ desensitization (30) (Figure 1). Myofilament proteins are the targets of nNOS that mediate relaxation in cardiac myocytes to decrease the myocardium in hypertensive heart. Exogenous NO donors ease myocardial relaxation through sGC and cGMP/PKG-dependent phosphorylation of cTnI and myofilament Ca2+ desensitization [75]. A recent report has shown that NO mimetics diminish myofilament Ca2+ sensitivity and contractility by causing the S-nitrosylation of many myofilament proteins containing actin, myosin, and troponin C (cTnC) [76]. These results suggest that phosphorylation and the myofilament proteins are the fundamental mechanisms that mediate the effects of nNOS in the heart. nNOS is considered as the isoform that is stated in the mitochondria to organize cardiac metabolism [76]. NO inhibits cytochrome c oxidase activity by competing with O2 and inhibits electron transfer of complex III or NADH-dehydrogenase function at the level of complex I and enhances mitochondrial formation of O2 −. Eventually, NO inhibits the mitochondrial respiration chain and diminish mitochondrial oxygen consumption [77-83]. In this respect, NO has been approved as a regulator of mitochondrial activity and metabolism. Even so, conditional overexpression of nNOS in the myocardium has related to enhanced nNOS in the mitochondria and a reduction in oxidative stress following myocardial infarction [51]. The modulation of oxidative stress by endogenous nNOS in diseased hearts can be a protective mechanism. Emerging evidence demonstrates that nNOS-derived NO plays leading roles in mitochondrial biogenesis [84,85], to maintain or enhance mitochondrial integrity and activity. For example, nNOS has been shown to be distributed to the nucleus through α-syntrophin via its PDZ domain in a variety of cells, containing myocytes [33,86]. Enhanced S-nitrosylation of nuclear proteins, containing cAMP response element-binding protein (CREB), in interacts with the promoter of the gene encoding peroxisome proliferator-activated receptor γ coactivator (PGC)-1α promoter, a central component of biogenesis and nuclear respiratory factor 1 [33]. NO has also included in cardiac energetics by impressing carbohydrate metabolism of mitochondria (Figure 2).
Clinical usage
Nitroglycerin has been used clinically in the treatment of CVD for more than 150 years. Enhanced acknowledgment of the mechanistic insights into NO signaling, the decomposition of NO, and the properties of NOSs modern technology allows different attitudes to enhance NO bioavailability in tissues for the desired responses as well. In principle, enhancement of NO and its signaling can be succeeded via three ways: enhance sources to contribute NO production, reduce NO metabolism/degradation, and stimulate downstream signaling of NO. Delivering nitrate and nitrite to step up systematic or local NO via nitrate–nitrite–NO and the nitrate–nitrite–NO–fatty acid pathways are arguably the most active area under investigation experimentally and in the clinic [10]. So far, some putative precursors of NO have been described and are developing. Dietary consumption of NO precursors is an efficient way of nitrate delivery; programming of a suitable diet regime for vulnerable populations will be necessary to diminish the cardiovascular risks the economic burden on national healthcare systems as well. The correlation between the daily consumption of nitrate and cardiovascular events is notable. For instance, high vegetable units received in Japanese historically recognised have low rates of CVD is related to greater circulating nitrate and nitrite [87], contrasted to those in the western world, where average daily nitrate intake ranges from 40–100 mg and 30–180 mg, respectively, and the rates of CVD are high [88,89]. Moreover, the use of “healthy” fats, as in the Mediterranean diet, in the form of unsaturated fatty acids, is useful in preventing the development of CVD and decreases the risk factors [90]. Especially, nitrite reduction to NO happens in the presence of hypoxia and acidosis, during physical exercise, at the time when the cardiac muscle needs NO. In rotation, supplementation of NO substrates, e.g. arginine, L-citrulline, and BH4, and inhibition of arginase and asymmetric dimethylarginine are the strategies to enhance NO via promoting NOS activity [11]. Statins and nebivolol or carvedilol exert antiadrenergic responses through the stimulation of the beta3-adrenergic receptor and increasing NOS production of NO [91–94]. Waning the generation of ROS use blockers of angiotensin-converting enzyme (ACE), angiotensin I type 1 receptor (AT1R), or NADPH oxidases (NOXs) or decreasing ROS by use antioxidants and scavengers are the assumed mechanisms to diminish NO “sink” and thus maintain or increase NO level [11]. As such, it is wrong only to assume that NO level can be enhanced by use ACE and AT1R inhibitors. The development of NOX inhibitors and specific ROS manipulating drugs that do not affect NOS protein should follow, and the effect of NOX and ROS on nNOS protein expression in the myocardium should be considered. Stimulation of the downstream signaling pathway of NO is a recovered strategy to target the effector proteins. The oral sGC stimulators, and atrial, brain, and C-type natriuretic peptides are in using to enhance cellular Cgmp [11]. Inhibition of a negative regulator of cGMP, PDE5 is another therapeutic approach to stimulate cGMP/PKG signaling [95-97]. Stimulation of the PKG-dependent pathway has been demonstrated to exert potent protective effects in a broad range of cardiovascular disease models, including hypertension, PAH, heart failure, hemolytic anemia, and infarct-reperfusion injury [95-99]. But, the application of the drugs in a large cohort of patients with CVD demonstrate responses to the treatment. Some validated ways have been developed to enhance systemic and local NO levels and are promising in mediating the beneficial effects in CVD. But, to translate the research innovations into the application to a large population, more research is necessary, with particular attention to the effectiveness of the diet and strategies of increasing nNOS and improving NO-effector interactions in CVD settings. The NO and NOSs regulate myocardial contraction, relaxation, and pathological signaling are advanced, but the changing paradigm in the myocardium is not offered. NO from sources supplying the NO in the myocardium, and effectiveness of NO is confirmed by regulation mechanisms containing daily consumption of NO precursors, nitrate from skeletal muscles, NO production through the entire salivary NO pathway and from NOSs as well as the abundance of target proteins. In general, NO regulates downstream effector proteins through three mechanisms (sGC/cGMP/PKG-dependent phosphorylation, S-nitrosylation, and trans-nitrosylation) and the numbers and types of effectors regulated by NO are diverse. As such, modification of these effectors by NO subsequently triggers an array of signaling cascades that lead to different physiological and pathological consequences. By and large, NO and its downstream signaling pathway exert high cardiovascular protection; but, research of NO and NOS that are feasible for CVD and therapeutic efficiency using an NO-dependent regime are still far from satisfactory [11].
- Arnold WP, Mittal CK, Katsuki S, Murad F (1977) Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci 74: 3203–3207. Link: https://goo.gl/6idhmD
- Furchgott RF, Zawadzki JV (1980) the obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376. Link: https://goo.gl/TZWJt1
- Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ et al. (1979) Relaxation of the bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosamine. J Cyclic Nucleotide Res 5: 211–24. Link: https://goo.gl/sNkDC3
- Massion PB, Feron O, Dessy C, Balligand JL (2003) Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398. Link: https://goo.gl/PEXJcT
- Shah AM, MacCarthy PA (2000) Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 86: 49–86. Link: https://goo.gl/zFc4Tw
- Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC (1999) Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci 96: 657–662. Link: https://goo.gl/4mXhRL
- Zhang YH, Jin CZ, Jang JH, Wang Y (2014) Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. J Physiol 592: 3189–3200. Link: https://goo.gl/7thRdD
- Zhang YH, Casadei B (2012) Sub-cellular targeting of constitutive NOS in health and disease. J Mol Cell Cardiol 52: 341–350. Link: https://goo.gl/sntu5g
- Omar SA, Webb AJ (2014) Nitrite reduction and cardiovascular protection. J Mol Cell Cardiol 73: 57–69. Link: https://goo.gl/vzcqJa
- Castiglione N, Rinaldo S, Giardina G, Stelitano V, Cutruzzolà F (2012) Nitrite and nitrate reductases: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 17: 684–716. Link: https://goo.gl/Z9MJFk
- Lundberg JO, Gladwin MT, Weitzberg E (2015) Strategies to increase nitric oxide signaling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641. Link: https://goo.gl/uVmYK5
- Doel JJ, Godber BL, Goult TA, Eisenthal R, Harrison R (2000) Reduction of organic nitrites to nitric oxide catalyzed by xanthine oxidase: possible role in the metabolism of nitrovasodilator. Biochem Biophys Res Commun 270: 880–885. Link: https://goo.gl/3FPGUK
- Zhang Z, Naughton DP, Blake DR, Benjamin N, Stevens CR et al. (1997) Human xanthine oxidase converts nitrite ions into nitric oxide (NO). Biochem Soc Trans 25: 524S. Link: https://goo.gl/nZY19J
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD et al. (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505. Link: https://goo.gl/XBiQWi
- Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M et al. (2007) Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754. Link: https://goo.gl/aMi3Er
- Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA et al. (2007) Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661. Link: https://goo.gl/pXNCag
- Petersen MG, Dewilde S, Fago A (2008) Reactions of ferrous neuroglobin and cytoglobin with nitrate under anaerobic conditions. J Inorg Biochem 102: 1777–1782. Link: https://goo.gl/3wjkR3
- Kozlov AV, Staniek K, Nohl H (1999) Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett 454: 127–30. Link: https://goo.gl/WNLLEq
- Kozlov AV, Dietrich B, Nohl H (2003) Various intracellular compartments cooperate in the release of nitric oxide from glycerol trinitrate in the liver. Br J Pharmacol 139: 989–997. Link: https://goo.gl/NNzd47
- Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL (2008) Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283: 17855–17863. Link: https://goo.gl/MjyQ37
- Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A et al. (2009) Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol 297: H2068–H2074. Link: https://goo.gl/ng8MgH
- Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE (2007) Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci 64: 96–103. Link: https://goo.gl/yt4Q5F
- Pennington JA (1998) Dietary exposure models for nitrates and nitrites. Food Control 9: 385–395. Link: https://goo.gl/G7ZWd8
- Piknova B, Park JW, Swanson KM, Dey S, Noguchi CT et al. (2015) skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 47: 10–16. Link: https://goo.gl/D2NTZh
- Percival JM, Anderson KN, Huang P, Adams ME, Froehner SC (2010) Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest 120: 816–826. Link: https://goo.gl/RWCi53
- Balligand JL, Feron O, Dessy C (2009) eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89: 481–534. Link: https://goo.gl/YQR5s5
- Villanueva C, Giulivi C (2010) Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med 49: 307–316. Link: https://goo.gl/Tv7V3L
- Goligorsky MS, Li H, Brodsky S, Chen J (2002) Relationships between caveolae and eNOS: everything in proximity and the proximity of everything. Am J Physiol Renal Physiol 283: F1–10. Link: https://goo.gl/3wCZZQ
- García-Cardeña G, Oh P, Liu J, Schnitzer JE, Sessa WC (1996) Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci 93: 6448–6453. Link: https://goo.gl/B2sokk
- Piech A, Massart PE, Dessy C, Feron O, Havaux X, et al. (2002) Decreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 282: H219–H231. Link: https://goo.gl/2L445x
- Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V et al. (2004) Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in the rat. Circulation 110: 2368–2375. Link: https://goo.gl/GfaLRM
- Jin CZ, Jang JH, Kim HJ, Wang Y, Hwang IC, et al. (2013) Myofilament Ca2+ desensitization mediates the positive lusitropic effect of neuronal nitric oxide synthase in left ventricular myocytes from the murine hypertensive heart. J Mol Cell Cardiol 60: 107–115. Link: https://goo.gl/hKVHpM
- Aquilano K, Baldelli S, Ciriolo MR (2014) nuclear recruitment of neuronal nitric oxide synthase by α-syntrophin is crucial for the induction of mitochondrial biogenesis. J Biol Chem 289: 365–378. Link: https://goo.gl/sBK2h5
- Jang JH, Kang MJ, Ko GP, Kim SJ, Yi EC, et al. (2015) Identification of a novel splice variant of neuronal nitric oxide synthase, nNOSβ, in myofilament fraction of murine cardiomyocytes. Nitric Oxide 50: 20–27. Link: https://goo.gl/96GQnU
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, et al. (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–339. Link: https://goo.gl/9JTW8V
- Damy T, Ratajczak P, Shah AM, Camors E, Marty I et al. (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363: 1365–1367. Link: https://goo.gl/zxar91
- Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C et al. (2006) Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha one subunit and reduced ischemia/reperfusion injury. Circ Res 98: 403–11. Link: https://goo.gl/FKWroQ
- Petroff MG, Kim SH, Pepe S, Dessy C, Marbán E, et al. (2001) Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol 3: 867–873. Link: https://goo.gl/ptTuEa
- Jian Z, Huilan Han, Zhang T, Puglisi J, Izu LT et al. (2014) Mechanochemotransduction during cardiomyocyte contraction is mediated by localized nitric oxide signaling. Sci Signal 7: ra27. Link: https://goo.gl/bmtqNq
- Wynia-Smith SL, Smith BC (2017) Nitrosothiol formation and S-nitrosation signaling through nitric oxide synthases. Nitric Oxide 63: 52–60. Link: https://goo.gl/Uqrk4w
- Murphy E, Kohr M, Menazza S, Nguyen T, Evangelista A, et al. (2014) Signaling by S-nitrosylation in the heart. J Mol Cell Cardiol 73: 18–25. Link: https://goo.gl/6wugxE
- Kohr MJ, Murphy E, Steenbergen C (2014) Glyceraldehyde-3-phosphate Dehydrogenase acts as a mitochondrial trans-S-nitrosyl rase in the heart. PLoS One 9: e111448. Link: https://goo.gl/rMKfrf
- Burgoyne JR, Eaton P (2009) Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent Cross-Talk to beta-adrenergic-like signaling. J Biol Chem 284: 29260–29268. Link: https://goo.gl/RiXuSt
- Benhar M, Forrester MT, Stamler JS (2009) Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10: 721–732. Link: https://goo.gl/WHmNPN
- Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: A current perspective. Trends Mol Med 15: 391–404. Link: https://goo.gl/kDhZRC
- Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, et al. (2008) Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res 102: 242–249. Link: https://goo.gl/5WcHxd
- Hammond J, Balligand JL (2012) Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling. J Mol Cell Cardiol 52: 330–340. Link: https://goo.gl/Wie9Pc
- Idigo WO, Reilly S, Zhang MH, Zhang YH, Jayaram R, et al. (2012) Regulation of endothelial nitric-oxide Synthase (NOS) S-glutathionylation by neuronal NOS: evidence of a functional interaction between myocardial constitutive NOS isoforms. J Biol Chem 287: 43665–43673. Link: https://goo.gl/76oxNV
- Jin CZ, Jang JH, Wang Y, Jang JH, Wang Y, et al. (2012) Neuronal nitric oxide synthase is up-regulated by angiotensin II and attenuates NADPH oxidase activity and facilitates relaxation in murine left ventricular myocytes. J Mol Cell Cardiol 52: 1274–1281. Link: https://goo.gl/ciUFTU
- Zhang YH, Dingle L, Hall R, Casadei B (2009) The role of nitric oxide and reactive oxygen species in the positive inotropic response to mechanical stretch in the mammalian myocardium. Biochim Biophys Acta 1787: 811–817. Link: https://goo.gl/ECg8nz
- Burkard N, Williams T, Czolbe M, Blömer N, Panther F, et al. (2010) Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation 122: 1588–1603. Link: https://goo.gl/w4Rgqx
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, et al. (2004) S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207. Link: https://goo.gl/npMQGC
- Pastore A, Piemonte F (2013) Protein glutathionylation in cardiovascular diseases. Int J Mol Sci 14: 20845–20876. Link: https://goo.gl/R98riz
- Capettini LS, Cortes SF, Gomes MA, Silva GA, Pesquero JL, et al. (2008) Neuronal nitric oxide synthase-derived hydrogen peroxide is a major endothelium-dependent relaxing factor. Am J Physiol Heart Circ Physiol 295: H2503–H2511. Link: https://goo.gl/3hdax8
- Capettini LS, Cortes SF, Lemos VS (2010) Relative contribution of eNOS and nNOS to endothelium-dependent vasodilation in the mouse aorta. Eur J Pharmacol 643: 260–266. Link: https://goo.gl/joEiTy
- Rabelo LA, Cortes SF, Alvarez-Leite JI, Lemos VS (2003) Endothelium dysfunction in LDL receptor knockout mice: a role for H2O2. Br J Pharmacol 138: 1215–1220. Link: https://goo.gl/gbMjZF
- Capettini LS, Cortes SF, Silva JF, Alvarez-Leite JI, Lemos VS (2011) Decreased production of neuronal NOS-derived hydrogen peroxide contributes to endothelial dysfunction in atherosclerosis. Br J Pharmacol 164: 1738–1748. Link: https://goo.gl/scCz2f
- Silva GC, Silva JF, Diniz TF, Lemos VS, Cortes SF (2016) endothelial dysfunction in DOCA-salt-hypertensive mice: the role of neuronal nitric oxide synthase-derived hydrogen peroxide. Clin Sci (Lond) 130: 895–906. Link: https://goo.gl/kKb91Y
- Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, et al. (2006) Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem 281: 21827–21836. Link: https://goo.gl/uDn17j
- Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, et al. (2007) Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317: 1393–1397. Link: https://goo.gl/kscGEc
- Kohr MJ, Davis JP, Ziolo MT (2009) Peroxynitrite Increases Protein Phosphatase Activity and Promotes the Interaction of Phospholamban with Protein Phosphatase 2a in the Myocardium. Nitric Oxide 20: 217–221. Link: https://goo.gl/zmA7HY
- Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, et al. (2009) Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP glycohydrolase I expression. J Biol Chem 284: 1136–1144. Link: https://goo.gl/7DmYYs
- Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, et al. (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118. Link: https://goo.gl/owDFj7
- Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, et al. (2009) Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J 30: 1142–1150. Link: https://goo.gl/tEVWF7
- Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, et al. (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res 92: e52–e59. Link: https://goo.gl/hCyVLh
- Wang Y, Youm JB,Jin CZ, Shin DH, Zhao ZH, et al. (2015) Modulation of L-type Ca2+ channel activity by neuronal nitric oxide synthase and myofilament Ca2+ sensitivity in cardiac myocytes from a hypertensive rat. Cell Calcium 58: 264–274. Link: https://goo.gl/6Sc8Yn
- Gonzalez DR, Beigi F, Treuer AV, Hare JM (2007) Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci 104: 20612–20617. Link: https://goo.gl/2qHKSg
- Wang H, Viatchenko-Karpinski S, Sun J, Györke I, Benkusky NA, et al. (2010) Regulation of myocyte contraction via neuronal nitric oxide synthase: the role of ryanodine receptor S-nitrosylation. J Physiol 588: 2905–2917. Link: https://goo.gl/knHXAw
- Burger DE, Lu X, Lei M, Xiang FL, Hammoud L et al. (2009) Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation 120: 1345–1354. Link: https://goo.gl/g3FMsf
- Cutler MJ, Plummer BN, Wan X, Sun QA, Hess D, et al. (2012) Aberrant S-nitrosylation mediates calcium-triggered ventricular arrhythmia in the intact heart. Proc Natl Acad Sci 109: 18186–18191. Link: https://goo.gl/j8Fp2G
- Núñez L, Vaquero M, Gómez R, Caballero R, Mateos-Cáceres P, et al. (2006) Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res 72: 80–89. Link: https://goo.gl/v5Xyvz
- Gómez R, Caballero R, Barana A, Amorós I, Calvo E et al. (2009) Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res 105: 383–392. Link: https://goo.gl/De9tqf
- Asada K, Kurokawa J, Furukawa T (2009) Redox- and calmodulin-dependent S-nitrosylation of the KCNQ1 channel. J Biol Chem 284: 6014–6020. Link: https://goo.gl/jB6x2b
- Bencsik P, Kupai K, Giricz Z, Görbe A, Huliák I, et al. (2008) Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S-nitrosylation of SERCA: the role of peroxynitrite. Br J Pharmacol 153: 488–496. Link: https://goo.gl/PjGXnG
- Layland J, Jian-Mei Li, Ajay M Shah (2002) Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol 540: 457–467. Link: https://goo.gl/5bzuSj
- Figueiredo-Freitas C, Dulce RA, Foster MW, Liang J, Yamashita AM, et al. (2015) S-Nitrosylation of Sarcomeric Proteins Depresses Myofilament Ca2+ Sensitivity in Intact Cardiomyocytes. Antioxid Redox Signal 23: 1017–1034. Link: https://goo.gl/iX9L7X
- Dedkova EN, Blatter LA (2006) mitochondrial calcium uptake stimulates nitric oxide and ROS production by mitochondria-specific nitric oxide synthase (mtNOS) in cat ventricular myocytes. Biophys J 90: 521. Link: https://goo.gl/miiLRk
- Erusalimsky JD, Moncada S (2007) Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol 27: 2524–2531. Link: https://goo.gl/XQeCcs
- Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, et al. (2013) Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759. Link: https://goo.gl/Uum1z2
- Welter R, Yu L, Yu CA (1996) the effects of nitric oxide on electron transport complexes. Arch Biochem Biophys 331: 9–14. Link: https://goo.gl/Gmrxqq
- Torres J, Darley-Usmar V, Wilson MT (1995) Inhibition of cytochrome c oxidase in turnover by nitric oxide: mechanism and implications for control of respiration. Biochem J 312: 169–173. Link: https://goo.gl/6b1SD4
- Grozdanovic Z (2001) NO message from muscle. Microsc Res Tech 55: 148–153. Link: https://goo.gl/WuoQYX
- Sarti P, Arese M, Forte E, Giuffrè A, Mastronicola D (2012) Mitochondrial and nitric oxide: chemistry and pathophysiology. Adv Exp Med Biol 942: 75–92. Link: https://goo.gl/tXcQQ2
- Wadley GD, Choate J, McConell GK (2007) NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol 585: 253–262. Link: https://goo.gl/VFYbej
- Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, et al. (2008) Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci 28: 2015–2024. Link: https://goo.gl/upr5oq
- Baldelli S, Lettieri Barbato D, Tatulli G, Aquilano K, Ciriolo MR (2014) The role of nNOS and PGC-1α in skeletal muscle cells. J Cell Sci 127: 4813–4820. Link: https://goo.gl/v5cuoSB
- Sobko T, Marcus C, Govoni M, Kamiya S (2010) Dietary nitrate in traditional Japanese foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide 22: 136–140. Link: https://goo.gl/ifAAjM
- Rathod KS, Velmurugan S, Ahluwalia A (2016) A ‘green' diet-based approach to cardiovascular health? Is inorganic nitrate the answer?. Mol Nutr Food Res 60: 185–202. Link: https://goo.gl/1Ra4Sx
- Hord NG, Tang Y, Bryan NS (2009) Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 90: 1–10. Link: https://goo.gl/7U6fLi
- Charles RL, Rudyk O, Prysyazhna O, Kamynina A, Yang J, et al. (2014) Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc Natl Acad Sc 111: 8167–8172. Link: https://goo.gl/ayvcek
- Vanhoutte PM, Gao Y (2013) Beta blockers, nitric oxide, and cardiovascular disease. Curr Opin Pharmacol 13: 265–273. Link: https://goo.gl/twbAmH
- Laufs U, Liao JK (1998) Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 273: 24266–24271. Link: https://goo.gl/C8xFkc
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, et al. (2000) The HMG-CoA reductase inhibitör simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med 6: 1004–1010. Link: https://goo.gl/eiFxjb
- Kosmidou I, Moore JP, Weber M, Searles CD (2007) Statin treatment and 3' polyadenylation of eNOS mRNA. Arterioscler Thromb Vasc Biol 27: 2642–2649. Link: https://goo.gl/ahm9Hw
- Salloum F, Yin C, Xi L, Kukreja RC (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–597. Link: https://goo.gl/XtxwHj
- Thadani U, Smith W, Nash S, Bittar N, Glasser S, et al. (2002) the effect of vardenafil, a potent and highly selective phosphodiesterase-5 inhibitor for the treatment of erectile dysfunction, on the cardiovascular response to exercise in patients with coronary artery disease. J Am Coll Cardiol 40: 2006–2012. Link: https://goo.gl/AM7m1w
- Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, et al. (2002) Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation 105: 2398–2403. Link: https://goo.gl/fgmmJR
- Raat NJ, Tabima DM, Specht PAC, Tejero J, Champion HC, et al. (2013) direct sGC activation bypass NO scavenging reactions of intravascular free oxy-hemoglobin and limits vasoconstriction. Antioxid Redox Signal 19: 2232–2243. Link: https://goo.gl/nFHnZx
- Stasch JP, Pacher P, Evgenov OV (2011) Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273. Link: https://goo.gl/n3rqbm
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
