Archives of Anatomy and Physiology
Boerhaavia diffusa L. Supplementation Attenuates Fluoride Induced Testicular Impairments in rats
Shashi A* and Imtiaza Khan
Cite this as
Shashi A, Khan I (2017) Boerhaavia diffusa L. Supplementation Attenuates Fluoride Induced Testicular Impairments in rats. Arch Anat Physiol 2(1): 027-035. DOI: 10.17352/aap.000008The present study investigated the effect of oral administration of sodium fluoride on testicular histopathology of rats and evaluated ameliorative effect of Boerhaavia diffusa L. Adult male rats weighing 100-150g were administered with sodium fluoride (NaF) at three different doses 100, 200 and 300 ppm/kg body weight, orally, daily for 40 days. At the end of the experimental period, half of the animals were sacrificed and their testis was removed. Remaining half rats were post treated with two doses (250 mg and 500 mg/kg body weight) of leaf extract of Boerhaavia diffusa L. for 20 days. Both light and scanning electron microscopy revealed dose dependent increase in testicular histopathological anomalies in NaF treated groups as compared to control. The normal histoarchitecture of seminiferous tubules was distorted. There was severe atrophy of seminiferous tubules as they were devoid of epithelium, with only sertoli cells and spermatogonia present within the depleted tubules. The spermatogenic cells showed degeneration and necrosis. There was severe tissue disruption, with absence of epithelial layers and complete cessation of spermatogenesis. The spermatozoa were absent in the tubules, only spermatocytes and spermatogonia were visible. The Leydig cell had scanty cytoplasm. The interstitial tissues were wide and congested. There was congestion and dilation of blood vessels. However, NaF groups post treated with leaf extract of Boerhaavia diffusa L. showed normal architecture of seminiferous tubules. The present study suggests that fluoride exerted disruptions to testicular histology was ameliorated by the administration of Boerhaavia diffusa L. extract.
Introduction
A large part of population in the industrialized world is exposed to variety of pollutants on daily basis, fluorides being one of them [1]. Besides immunotoxic, neurotoxic and cytotoxic effects induced by fluoride, the adverse influence of fluoride on the reproductive system has become a major concern in many countries. The importance of reproductive health to offspring developments has prompted epidemiological investigations of the apparent connection between excessive fluoride exposure to male fertility and low birth rates [2].
Involvement of the reproductive organs due to fluorosis in animals had also been studied extensively. Fluoride consumption for a longer period of time has many pathological effects as a result of increased oxidative stress on soft tissues like muscle, liver, gastrointestinal tract in addition to the reproductive and endocrine organs by the property of simple diffusion. Several clinical investigations and animal experiments suggested that fluoride has adverse impacts on male reproductive function [3,4], such as spermatogenesis and steriodogenesis [5], alterations in the epididymis and accessory reproductive glands and testicular cell cycle [6]. Moreover, high doses of fluoride could result in apoptosis of leydig cells [7], and vascular dystrophy in seminal cells and necrosis in mice [8].
Due to exposure to environmental pollutants and physical stress, the rate of male infertility has been increasing, which has become a very serious social problem. Boerhaavia diffusa L. is an important medicinal plant much used in Ayurveda and Unani medicines and in other traditional medicines in many parts of the world. Each part of this plant has a different therapeutic value. This plant rejuvenates liver, male reproductive system and other organ system, aphrodisiac, increases libido, erection and quality and quantity of semen [9]. Therefore, the present study was conducted to assess the toxic effects of fluoride on testicular histology and its alleviation by B. diffusa.
Materials and Methods
Young male Wistar rats weighing between 100-150 g were housed in polypropylene cages with stainless steel grill tops and fed with standard rat pellet diet (Hindustan Lever Limited, India) and maintained in an air conditioned animal house facility with controlled temperature (22-25ºC), 12h light/dark cycle and humidity. The water was given ad libitum. The experiments were performed under the approval of Instituitional animal ethic committee, Punjabi University Patiala (Approval number 107/99/CPCSEA-2012-11).
Preparation of plant extract
The leaf extract of B. diffusa was prepared by the method of Narendhirakannan, et al. [10].
Experimental design
After acclimatization to environment for one week, the animals were randomly divided into six groups with twelve animals in each group. The first group served as normal control administered with 1 ppm deionized water/kg b.w./day. The remaining three experimental groups received 100, 200 and 300 ppm sodium fluoride (NaF)/kg b.w./day. All the treatments were given daily by oral gavage for 40 days. Remaining half rats were post treated with B. diffusa (250 and 500 mg/kg b.w.) for next 20 days. Last two groups were kept as positive control I and positive control II administered with 250 and 500 mg/kg b.w./ day B. diffusa leaf extract, respectively. At the end of the experimental period, rats were fasted overnight and sacrificed and testis was removed, blotted free of blood, and processed for light and scanning electron microscopy.
Histopathological study
Light microscopy: After the completion of experimental regimen, the rats were fasted overnight and were excised under ether anesthesia and the reproductive organ (testis) were removed, blotted free of blood, fixed in Bouin’s fluid for 24 hours. The tissue was dehydrated in graded series of alcohol followed by tertiary butyl alcohol, cleared in amyl acetate, processed for paraffin embedding and 7µ thin paraffin sections were stained with haematoxylin and eosin [11]. Histopathological changes were studied under research binocular microscope and subsequently micro photographed.
Electron microscopy: After fixation in Karnovsky’s fixative, the specimens were washed in 0.1 M phosphate buffer and then fixed for 1 hr in 0.5% osmium tetraoxide. After few washes in 0.1 M phosphate buffer, the specimens were dehydrated through ascending grades of alcohol. After critical point drying followed by coating with gold pellets, the tissue were examined under a scanning electron microscope- JSM(6100) JEOL [12].
Results
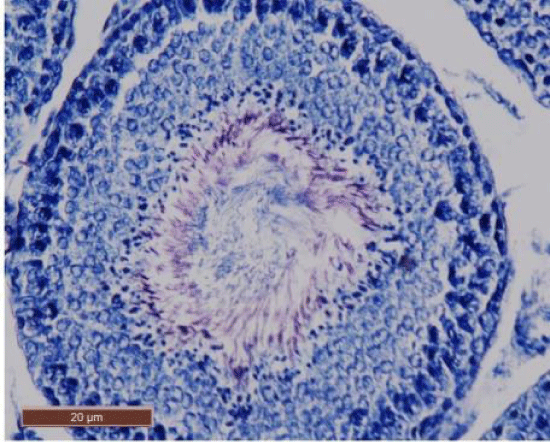
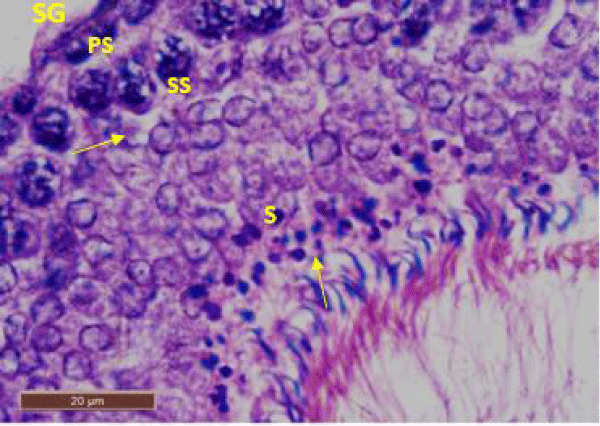
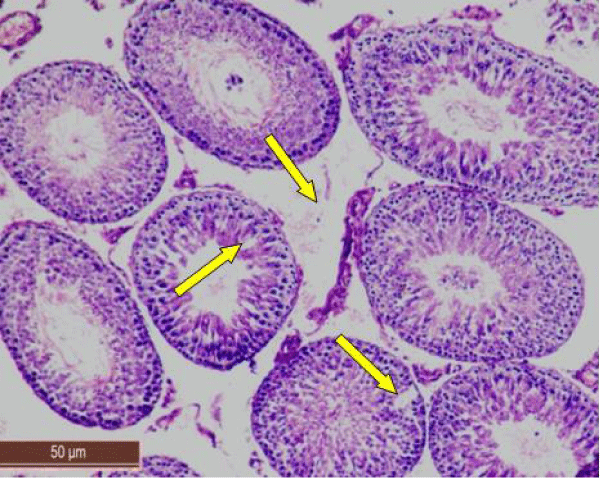
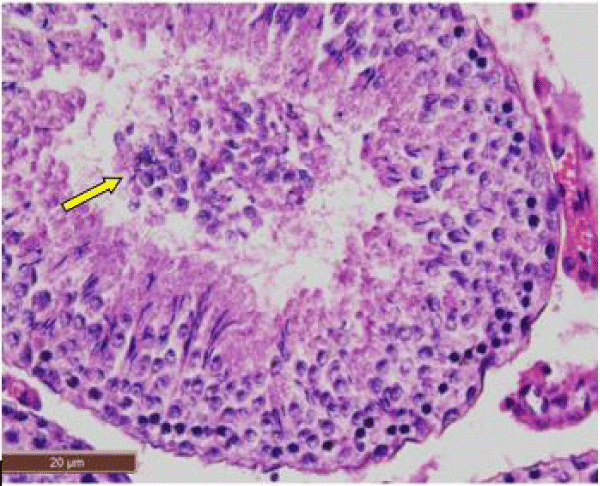
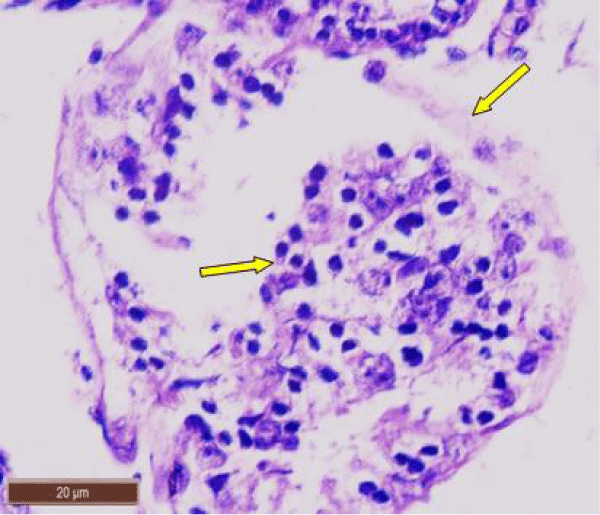
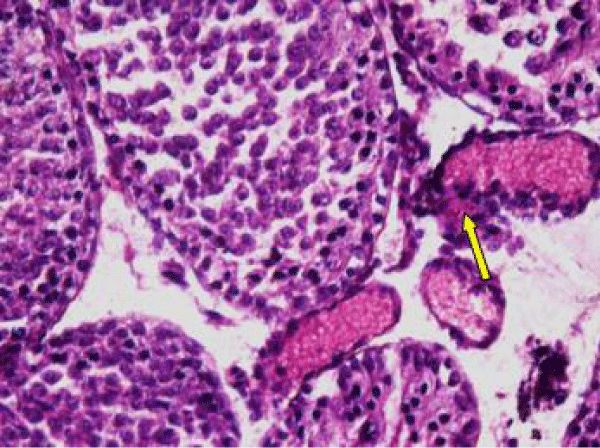
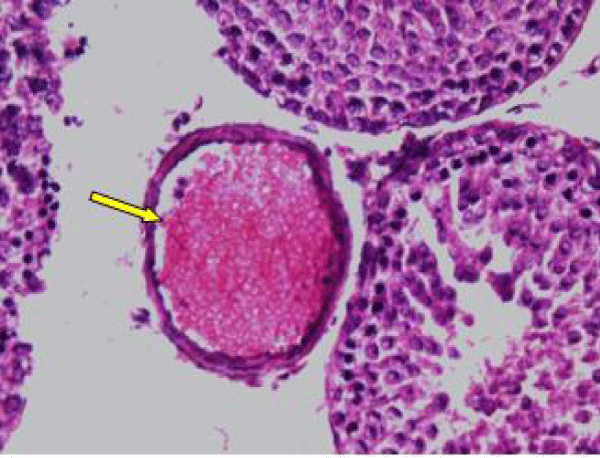
In control rat, the seminiferous tubules were surrounded by dense interstitium. The seminiferous tubules contain stratified germinal epithelium, which has two distinct populations of cells; the spermatogenic cells and the sertoli cells (Figure 1a). The spermatogonia were adhered to the basement membrane with their dark nuclei, primary spermatocytes were the largest cells, the spermatids appeared smaller than primary spermatocytes and lying near the lumen. The lumen enclosed large number of sperms and some of these sperms were attached to the apex of Sertoli cells (Figure 1b). Sertoli cells were distributed at intervals between spermatogenic cells. Sertoli cells appeared as tall cells extending from the basal membrane to the lumen. Both Sertoli cells and spermatogonia were seen resting on the basement membrane. The interstitial spaces contained delicate loose connective tissue and Leydig cells (Figure 1c).
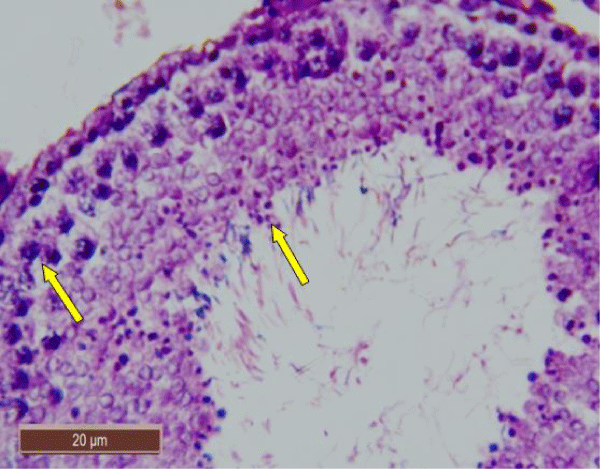
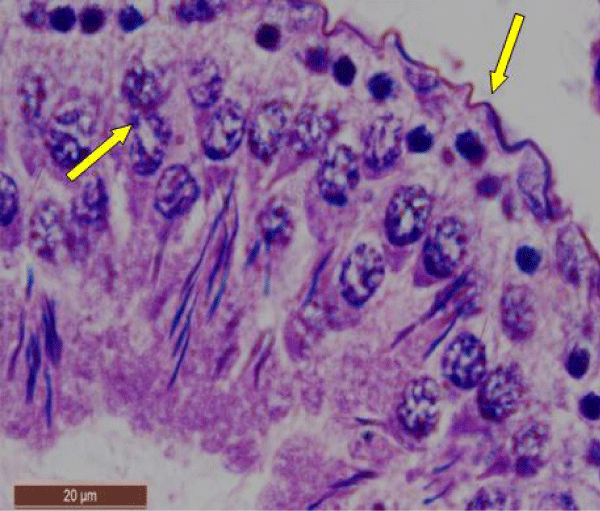
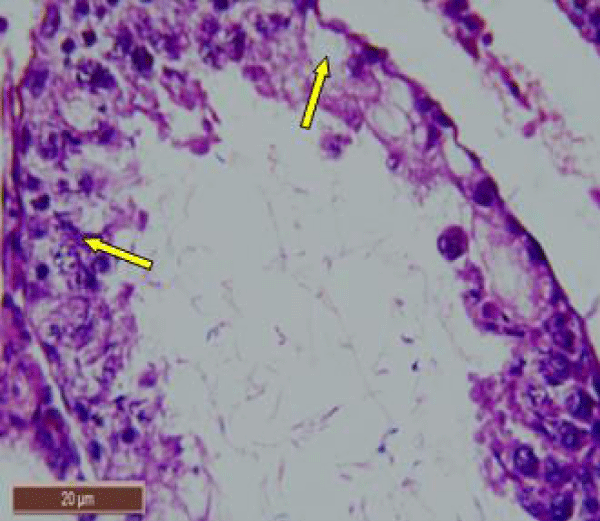
In rats treated with 100 ppm NaF, seminiferous tubules showed mild deterioration with irregularly shaped and disarranged epithelial layers (Figure 2a,b). The spermatogenic cells sloughed off into the interstitium (Figure 2c). Some spermatids and spermatozoa were observed in the lumen of the tubules (Figure 2d).
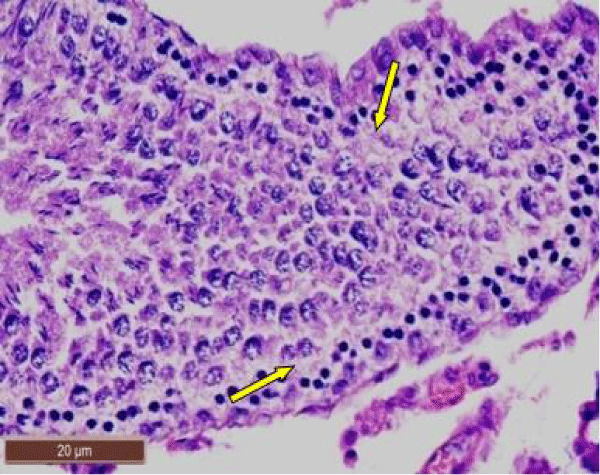
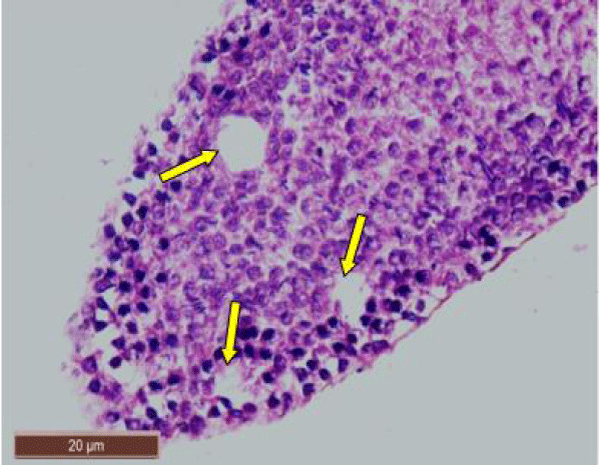
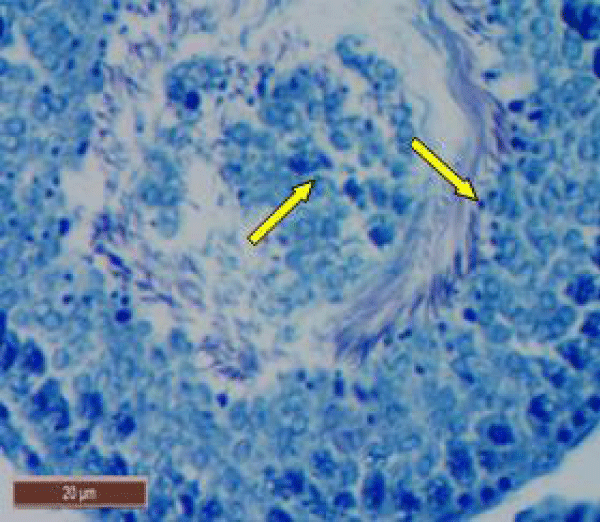
In rats treated with 200 ppm NaF, there was disruption of the germinal epithelium, alongwith loss of spermatogenic cells, spermatocytes and spermatids. Tubular lumen had a few scattered clusters of spermatozoa (Figure 3a). The exfoliation of germ cells (Figure 3b), pyknotic nuclei (Figure 3c), and focal areas of intraepithelial vacuolization (Figure 3d), were also observed.
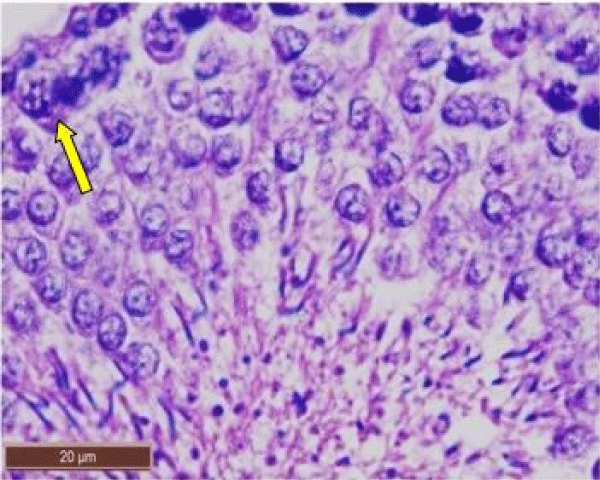
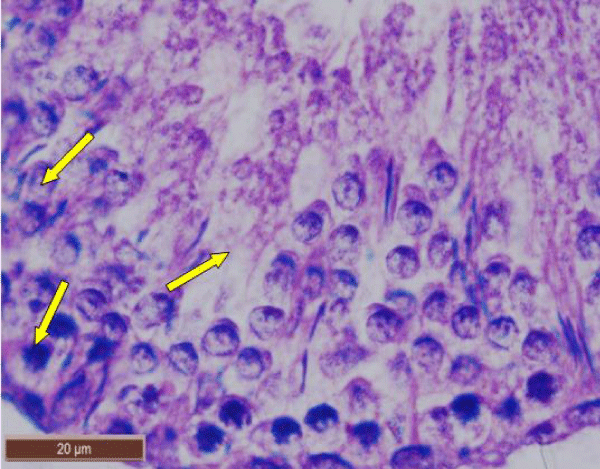
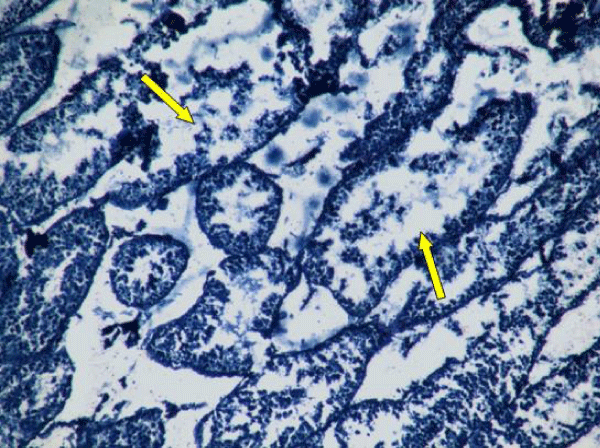
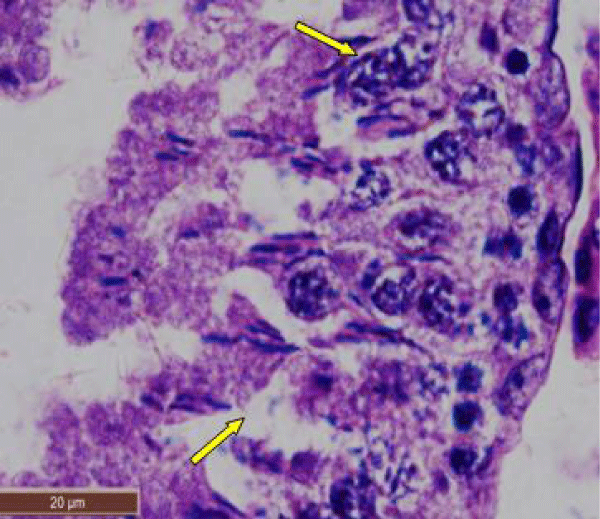
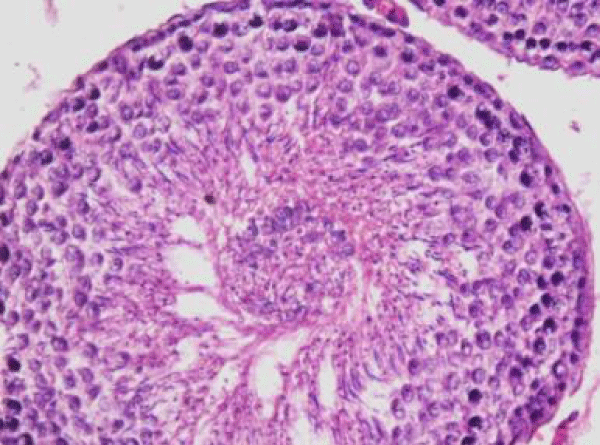
In rats treated with 300 ppm NaF, the seminiferous tubular wall was irregular (Figure 4a), and collapsed (Figure 4b). The basement membrane was thickened (Figure 4c), and ruptured (Figure 4d). The seminiferous tubule showed severe edema separating the developing germ cells and with apoptotic cells (Figure 4e). There was severe atrophy of seminiferous tubules as they were devoid of epithelium, with only sertoli cells and spermatogonia present within the depleted tubules. The spermatogenic cells showed degeneration and necrosis. Necrosis was in the form of pale or vacuolated cytoplasm, pyknotic nuclei and chromatin fragmentation (Figure 4f).
There was severe tissue disruption, with absence of epithelial layers and complete cessation of spermatogenesis. The spermatozoa were absent in the tubules, only spermatocytes and spermatogonia were visible. There was denudation of spermatocytes (Figure 4g), disintegration of Sertoli cells (Figure 4h).
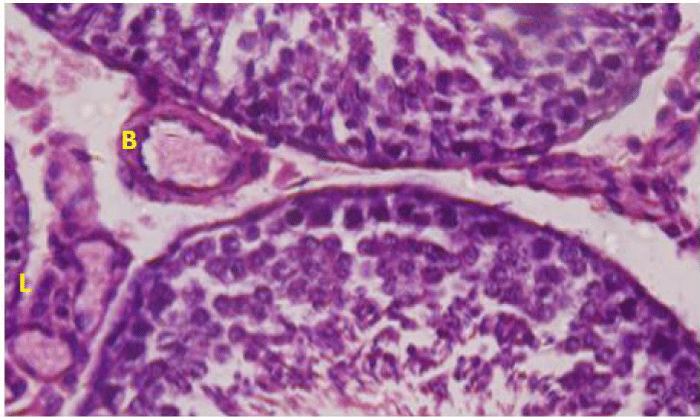
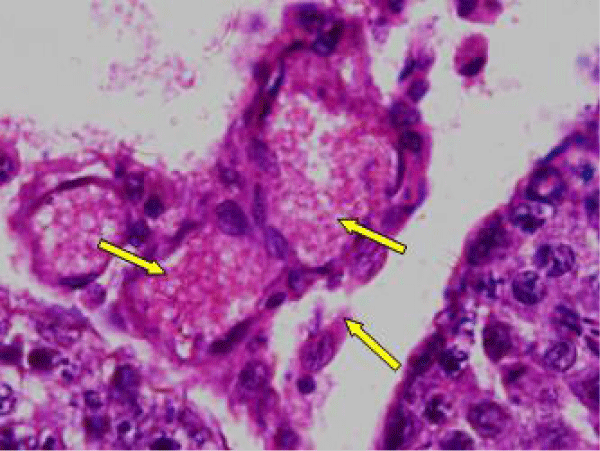
The Leydig cell had scanty cytoplasm (Figure 4i). The interstitial tissues were wide and congested (Figure 4j). There was congestion and dilation of blood vessels (Figure 4k).
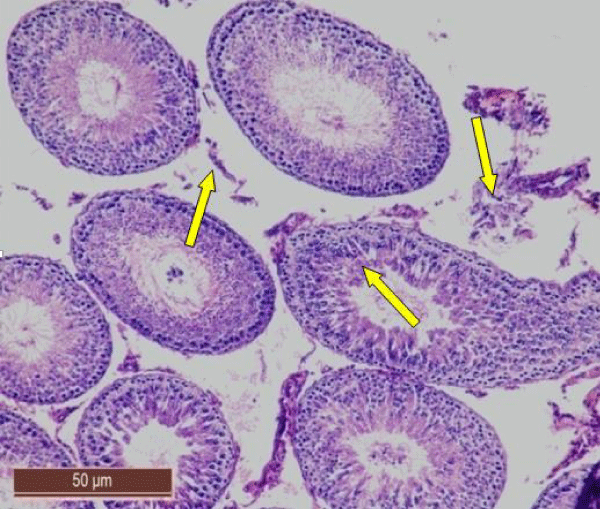
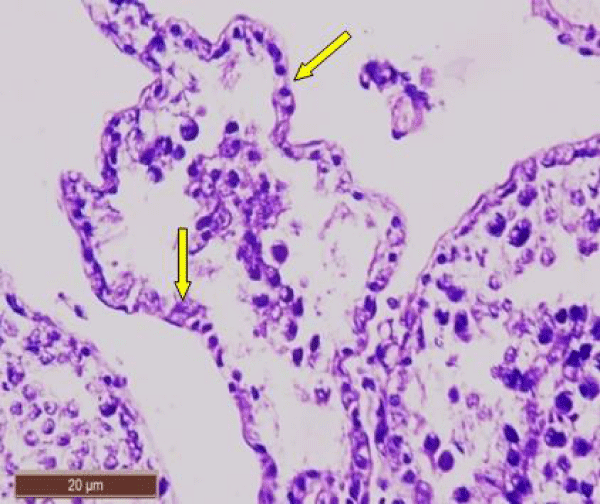
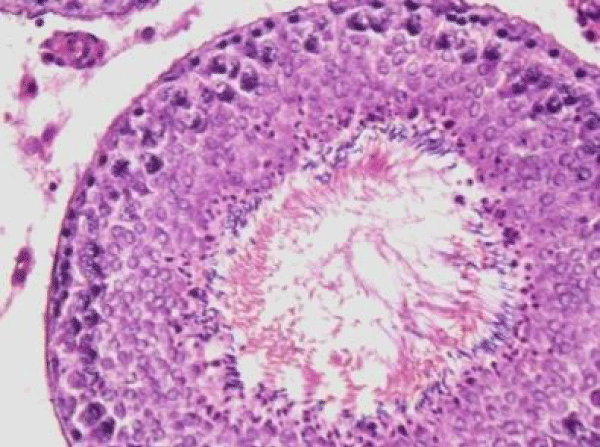
Nearly normal testicular structure was observed when rats were post treated with B. diffusa leaf extract. The post treatment of fluorotic rats with 250 mg/kg bw of leaf extract caused a slight increase in spermatogenic cell density specially in spermatids and spermatozoa. Outline of seminiferous tubule was improved (Figure 5).
While post treatment of fluorotic rats with 500 mg/kg bw of leaf extract of B. diffusa caused marked increase in the layer of spermatogenic cells. Spermatogonia and Sertoli cells were seen resting on the basement membrane followed by primary spermatocytes, early and late spermatids were nearly more or less similar to the control group (Figure 6).
The presence of degenerating spermatogenic cells, pyknotic nuclei and vacuolation were less in B. diffusa leaf extract treated groups.
Scanning electron microscopic observations
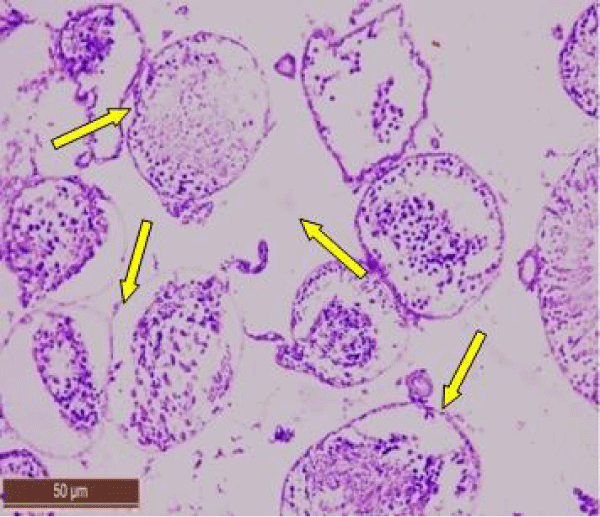
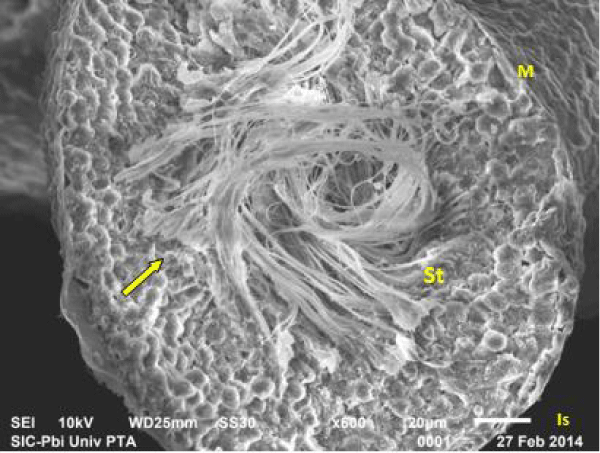
Scanning electron micrograph of control rat showed that the outer surface of the seminiferous tubule was covered with single layer of flat myoid cells, the nuclei of which appeared as small bulges. The myoid cells were arranged in a continuous monolayer (Figure 7a). Germ cells layers were visible. The tubular lumen filled was filled with flagella of spermatozoa (Figure 7b).
Scanning electron micrograph of testicular sections of rats treated with 100 and 200 ppm NaF showed narrow seminiferous tubule having irregular outlines (Figure 8a), with exfoliated germ cells in the lumen (Figure 8b). The number of spermatozoa was reduced (Figure 8c).
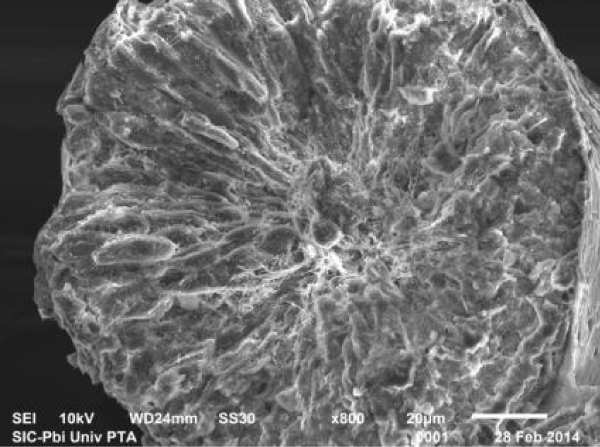
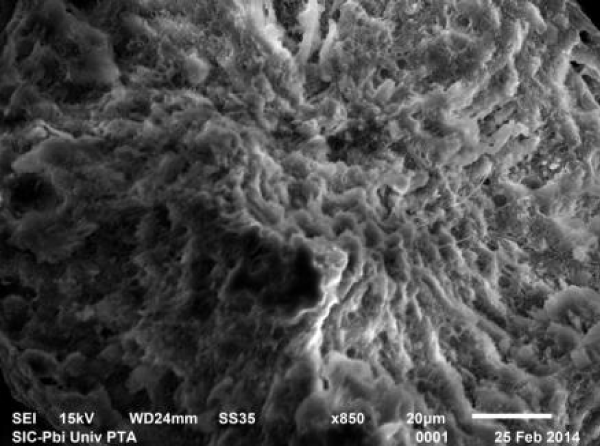
In rats treated with 300 ppm NaF scanning electron micrograph of seminiferous tubule showed many cavities (Figure 9a), and depletion of the germinal epithelial layers (Figure 9b), with decreased number of spermatozoa in the lumen (Figure 9c). Empty seminiferous tubules were also visible (Figure 9d).
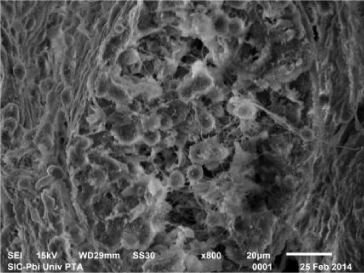
In fluorotic rats post treated with 250 mg/kg bw of B. diffusa leaf extract, scanning electron micrograph showed an improved outline of the seminiferous tubule. There was marked increase in the number of layers of the spermatogenic cells and increase in the number of spermatozoa (Figure 10).
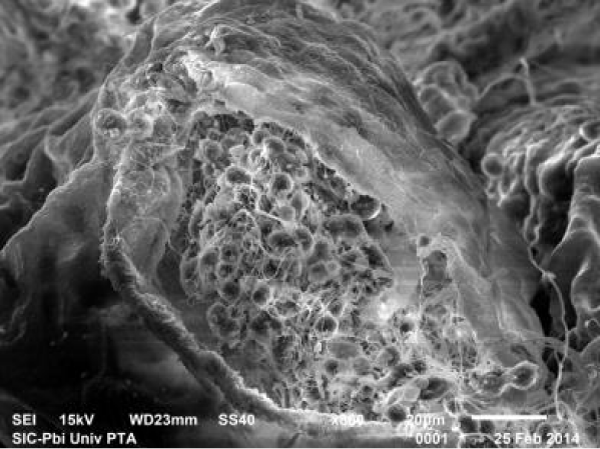
The fluorotic rats post treated with 500 mg/kg bw of B. diffusa leaf showed a significant improvement of testicular structure. The seminiferous tubules were well defined and lined by several layers of spermatogenic cells. The tubular lumen was filled with spermatozoa (Figure 11).
Discussion
In the present study, the microscopic examination of the testes of the control rats showed normal structure and normal spermatogenesis with different stages of differentiation and maturation. By contrast, the NaF treated group animals showed many pathological alterations in testis including necrosis and a prominent decline in the number of spermatogonial cells resulting in epithelial disorganization [13], and absence of all internal layers except scattered cells of spermatogonia layer along the basement membrane [14], leading to complete cessation of spermatogenesis and the tubular lumen showed a few scattered clusters of spermatozoa with luminal cellular debris [15], alongwith vaccular degeneration of the spermatogenic and sertoli cells as well as leydig cells necrosis [16]. Moreover, there was thick and irregular basement membrane [8]. In addition to, marked inflammatory infiltration in the interstitial tissue of the seminiferous tubules was observed. Congestion of interstitial blood vessel and thickening of tunica albuginia was also observed [17].
The findings on the altered histoarchitecture of testis are in accordance with the study of Sakr and Azab [18], and Manna et al. [19].
Wan et al. [20]. Also recorded that NaF (150mg/L of drinking water) caused disorganization, denudation and reduction in germinal epithelial cells of the seminiferous tubules and an accompanying absence of sperm in the lumina in histological sections of rats. Sekhar et al. [21]. Found that cypermethrin and NaF - treated mice testes exhibited clumped spermatozoa, vacuolation, severe necrosis and degenerative changes with increased lumen of seminiferous tubules. Beside these degenerative changes in spermatids, atrophied seminiferous tubules, necrotic changes in theca albuginea and scattered spermatids were also reported. Feng et al. [22], observed that on administration of 2.5 g/L NaF resulted in degeneration of seminiferous epithelium and depletion of sperm cells.
On the other side, the microscopic examination of the rats post treated with B. diffusa leaf extract revealed an improvement in histological picture of fluoride toxicity of testis sections. The later showed normal seminiferous tubules epithelium with distinct nuclei and mature sperm bundles in their lumen, with normal basement membrane as well as normal interstitial cells of Leydig cells and lack of congestion. All along the basement membranes, in each seminiferous tubule, spermatogonia were arranged in concentric layers (typically representing the zone of mitosis), followed by similar concentric layers of spermatocytes immediately inward to the spermatogonial layers (representing the zone of meiosis), while the core area contained differentiating spermatozoa (identified as the zone of spermiogenesis).
Thus, histopathological results of the present study confirmed the observations of previous authors in mice and rabbits [23,24]. Moreover, the NaF exposure showed severe loss of Leydig cells as a result of the oxidative stress that affected on steroidogenesis and testicular apoptosis [25].
The necrosis of seminiferous tubules was in consonance with the study of Yang et al. [26]. Who explained that fluoride stimulated free radicals which increased lipid peroxide levels, and decreased the activities of glutathione peroxidase and ATPase in testis and epididymis that picked up by mitochondria producing swelling and distortion of mitochondrial cristae, uncoupled energy metabolism, inhibited cellular respiration, and altered calcium kinetics, Moreover, Agha et al. [27], suggested that the oxidative stress results from the loss of equilibrium between oxidative and antioxidative mechanisms that can produce DNA fragmentation, resulting in apoptosis. The same finding was attributed by Shashi et al. [28], who explained that fluoride toxicity leads to loss of selective permeability of the cell membrane, resulting in dilatation of cytoplasmic component secondary to intracellular fluid and electrolyte redistribution.
Sertoli cell degeneration related to decreased sperm quality and abnormal spermatozoa has also been reported [29], which supported our observations, in addition to the apoptotic appearance of sertoli cells which are essential for nourishment. Yang et al. [26], documented that the severe irritation of fluoride ions on the tissue parenchyma may lead to the infiltration of inflammatory cells in testis and epididymis that causes apoptosis and necrosis of germinal cells.
The present study demonstrated that exposure to NaF caused histopathological changes in the testes that are indicative of changes in spermatogenesis function, especially in the seminiferous tubule. Furthermore, normal testicular structure and the maintenance of its internal microenvironment are important for sperm production and for maintaining good quality of sperms. By post treatment with B. diffusa leaf extract the testicular histology was resumed to normal architecture.
- Chouhan S, Flora SJ (2010) Arsenic and fluoride: two major ground water pollutanats. Indian J Exp Biol 48: 666-678. Link: https://goo.gl/HA8nk4
- Sun Z, Wang B, Niu R, Zhang JH, Wang JD, et al. (2009) Decreased sperm hyperactivation and low catsper1 expression in mice exposed to fluoride. Fluoride 42: 167-173. Link: https://goo.gl/dhiFcq
- El-Hallawany HA, El-Metwally AE (2011) Toxopathological studies on the effect of fluoride pollution on male and female fertility in adult rabbits. Assiut Vet Med J 57: 376-396.
- Sahu SK, Mishra DN, Pradhan S, Prusti JS, Panda SK, et al. (2015) Fluoride Induced Histopathological Changes in Liver of Albino Rabbit - An Experimental Study. Int J Pharm Sci Rev Res 30: 184-188. Link: https://goo.gl/jMRgVc
- Pushpalatha T, Srinivas M, Reddy PS (2005) Exposure to high fluoride concentration in drinking water will affect spermatogenesis and steroidogenesis in male albino rats. Biometals 18: 207-212. Link: https://goo.gl/UqH7t2
- Huang C, Niu R, Wang J, China S (2007) Toxic effects of sodium fluoride on reproductive function in male mice. Fluoride 40: 162-168. Link: https://goo.gl/yxYrhT
- Song GH, Wang RL, Chen ZY, Zhang B, Wang HL, et al. (2014) Toxic effects of sodium fluoride on cell proliferation and apoptosis of Leydig cells from young mice. J Physiol Biochem 70: 761-768. Link: https://goo.gl/cp2YAS
- Poesina ND, Balalau C, Barca M, Ion I, Baconi D, et al. (2013) Testicular histopathology changes following sodium fluoride administration in mice. Rom J Morphol Embryol 54: 1019-1024. Link: https://goo.gl/DvKL35
- Santhosha D, Ramesh A, Prasad MS, Kumar SD, Kumar PB, et al. (2011) Punarnava- A review. Res J Pharma Biol Chem Sci 2: 427-436. Link: https://goo.gl/McBEX1
- Narendhirakannan RT, Subramanian S, Kandaswamy M. (2006) Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin induced diabetes in experimental rats. Clin Exp Pharmacol Physiol 33: 1150-1157. Link: https://goo.gl/YvqaZ4
- Drury AR, Wallington EA (1980) Carleton's histological techniques. 5th edn. Oxford University Press. London 140. Link: https://goo.gl/58uPDD
- Flegler SL, Heckman JW, Klomparens KL (1993) Scanning and transmission electron microscopy. An introduction. Oxford University Press, New York.
- Wei R, Luo G, Sun Z, Wang S, Wang J (2016) Chronic fluoride exposure-induced testicular toxicity is associated with inflammatory response in mice. Chemosphere 153: 419-425. Link: https://goo.gl/v1HzSm
- Elesawy BH, Alghamdy AARN, El-Askary A, Salih MM (2016) Evaluation of the effect of vitamin D on sodium fluoride-induced toxicity in reproductive functions of male rabbits. National J Physiol Pharma Pharmacol 6: 222-225. Link: https://goo.gl/Kir9cq
- Saumya SM, Basha PM (2017) Fluoride exposure aggravates the testicular damage and sperm quality in diabetic mice: protective role of ginseng and banaba. Biol Trace Elem Res 177: 331-344. Link: https://goo.gl/eSMe5W
- Rao MV, Bhatt RN (2012) Protective effect of melatonin on fluoride-induced oxidative stress and testicular dysfunction in rats. Fluoride 45: 116-124. Link: https://goo.gl/bUUr6K
- El-Metwally AE, El-Hallawany HA, Ali AH (2017) Immunohistopathologic study on the ameliorative effect of propolis against fluoride cytotoxicity on rabbit buck fertility. AJVS 53: 156-166. Link: https://goo.gl/2K9RzZ
- Sakr SA, Azab AE (2001) Effect of pyrethroid inhalation on the testis of albino rat. Pak J Biol Sci 4: 498-500. Link: https://goo.gl/vdUWsb
- Manna S, Bhattacharya D, Basak DK, Mandal TK (2004) Single oral dose toxicity study of a-cypermethrin in rats. Indian J Pharmacol 36: 25-28. Link: https://goo.gl/HaAUQk
- Wan SX, Zhang JH, Wang JD (2006) Effects of high fluoride on sperm quality and testicular histology in male rats. Fluoride 39: 17-21. Link: https://goo.gl/c1szBi
- Sekhar PR, Savithri Y, Kishore S, Jayasankar A, Rao KJ (2011) Synergistic effect of sodium fluoride mad cypermethrin on somatic index and histopathology of albino mice testis. Fluoride 44: 103-111. Link: https://goo.gl/8ryJ9Q
- Feng D, Huang H, Yang Y, Yan T, Jin Y, et al. (2015) Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats testis. Mutation research 792: 35-45. Link: https://goo.gl/xHifhF
- Tiwari S, Pande RK (2011) Histopathological studies on the effect of sodium fluoride on reproductive organs and body weight of male albino rats. Int J Sci Innovation Discoveries 1: 294-302. Link: https://goo.gl/BVEk97
- Ahmad KR, Nauroze T, Raees K, Abbas T, Kanwal M, et al. (2012) protective role of Jambul (Syzgium Cumini) fruit-pulp extract against fluoride-induced toxicity in mice testis: a histopathological study. Fluoride 45: 281-289. Link: https://goo.gl/sDQUmX
- Falih EB, Ibrahim ZI (2016) Toxicopathologic Effects of Sodium Fluoride on Male's Reproductive System of Rabbits. Veterinary Sci 6: 435-438. Link: https://goo.gl/5MN9Hz
- Yang KD, Liu SH, Ying CJ (2002) Study on antagonistic effects of selenite on fluoride induced impairments of testis and epididymis in rats. Chung-Kuo, Kung Kung, Wei, Sheng (China Public Health) 18: 427-29. Link: https://goo.gl/F1N93x
- Agha FE, El-Badry MO, Hassan DAA, Abd Elraouf A (2012) Role of Vitamin E in combination with methionine and L- carnosine against sodium fluoride-induced hematological, biochemical, DNA damage, histological and immunohistochemical changes in pancreas of albino rats. Life Sci. J 9: 1260-1275. Link: https://goo.gl/zLvaDv
- Shashi A, Sharma N, Bhardwaj M (2010) Pathological evaluation of pancreatic exocrine glands in experimental fluorosis. Asian Pacific J Trop Med 3: 36-40. Link: https://goo.gl/pVF6g9
- Ozmen O, Mor F (2012) Apoptosis in adult rabbit testes during subacute endosulfan toxicity. Pesticide Biochem and Physio 102: 129-133. Link: https://goo.gl/9uxzav
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.







 Save to Mendeley
Save to Mendeley
