Archives of Anatomy and Physiology
Sekar’s DISH10 (Deep Inspiration, Squeeze & Hold for 10 seconds) Maneuver- A Novel, Non-invasive and Cost-effective Treatment for Postdural Puncture Headache – A Comparative Cohort Study
Kartik Sonawane1*, Chelliah Sekar2, Hrudini Dixit3 and Tuhin Mistry1
2Senior Consultant, Department of Anesthesiology, Ganga Medical Centre & Hospitals, Pvt. Ltd., Coimbatore, Tamil Nadu, India
3Fellow in Regional Anesthesia, Department of Anesthesiology, Ganga Medical Centre & Hospitals, Pvt. Ltd., Coimbatore, Tamil Nadu, India
Cite this as
Sonawane K, Sekar C, Dixit H, Mistry T (2021) Sekar’s DISH10 (Deep Inspiration, Squeeze & Hold for 10 seconds) Maneuver- A Novel, Non-invasive and Cost-effective Treatment for Postdural Puncture Headache – A Comparative Cohort Study. Arch Anat Physiol 6(1): 008-018. DOI: 10.17352/aap.000017Introduction: Postdural puncture headache (PDPH) is one of the iatrogenic complications of the neuraxial blockade. Its incidence has steadily declined with advances in anesthesia techniques, improved knowledge of pathophysiology, and the implementation of preventable measures. However, it has the potential to cause significant morbidity in affected individuals. This article introduces a new non-invasive and cost-effective treatment for PDPH termed DISH10 (Deep Inspiration, Squeeze & Hold for 10 seconds) maneuver. It also describes the essential steps involved in the DISH10 maneuver and discusses various biomechanics associated with these steps. We hypothesize that the DISH10 maneuver hastens spontaneous recovery by increasing intrathoracic and intraabdominal pressure and provides quick relief.
Methods: This comparative cohort study includes 100 PDPH patients in three years, from January 2018 to March 2021. This study is divided into two groups. Group 1 included a prospective case series of 50 patients of PDPH treated with DISH10 maneuver. Group 2 included a retrospective cohort of 50 patients of PDPH treated with conventional conservative management with or without sphenopalatine ganglion block (SPGB). The demographics, type of neuraxial anesthesia, size/type of spinal needle, time to develop headache, and time to outcome were noted.
Results: The incidence of PDPH was higher with 25G spinal needles (Quincke) in both the groups (82% in DISH10 and 74% in group 2) than with 27G spinal needles (Whitacre). The median of time to outcome (time to make patients symptom-free) with DISH10 maneuver was significantly lower (7 hours) than the conservative group (48 hours). All 50 patients in Group 1 (case series) became symptoms-free and ready to discharge within 24 hours of commencement of the DISH10 maneuver.
Conclusion: The DISH10 maneuver has shown better results than conventional conservative management with or without SPGB in terms of treatment duration, time to discharge, and total hospital stays, making the DISH10 maneuver a cost-effective option.
Abbreviations
PDPH: Postdural Puncture Headache; SPGB: Sphenopalatine Ganglion Block; DISH10: Deep Inspiration Squeeze and Hold for 10 Seconds; SA: Spinal Anesthesia; RA: Regional Analgesia; CSF: Cerebrospinal Fluid; VAS: Visual Analog Scale; FG: Functional Grading; EBP: Epidural Blood Patch; VM: Valsalva Maneuver; CPP: Cerebral Perfusion Pressure; MAP: Mean Arterial Pressure
Introduction
August Bier first described postdural puncture headache (PDPH) in 1899 after self experiencing it following spinal anesthesia (SA) [1]. PDPH is one of the common complications of the neuraxial blockade, with the incidence varies from 0.3%-20% following SA and about 70% after an accidental dural puncture [2]. Symptoms develop within 48 hours to 5 days after the procedure. In the modern anesthesia practice, the rates of PDPH following SA have steadily declined, from an incidence exceeding 50% in Bier’s time to around 10% in the 1950s [3], until currently a rate of 1% or less can be reasonably expected.
Many questions still remain unanswered despite various management strategies (conservative, non-pharmacological, pharmacological, and interventional therapies) described in the literature to treat PDPH. Few regional anesthesia (RA) techniques like erector spinae plane block at T4 [4], greater occipital nerve block [5], and sphenopalatine ganglion block (SPGB) [6] provided symptomatic relief. Such modalities are either partially effective, time-consuming, expensive, difficult to perform, and/or associated with complications. One of the authors (CS) attempted to circumvent all these issues and devised a novel, non-invasive and cost-effective maneuver called Sekar’s DISH10 (Deep Inspiration, Squeeze & Hold for 10 seconds) maneuver.
We hypothesize that the DISH10 maneuver maximizes intrathoracic and intra-abdominal pressure sufficiently to engorge epidural vessels. That leads to the increased epidural pressure, which stops cereborspinal fluid (CSF) leakage and restores its homeostasis. Thus, the DISH10 maneuver potentially hastens spontaneous recovery by providing quick relief and increasing patient satisfaction. We have successfully treated many PDPH patients (since 2018) after incorporating the DISH10 maneuver into our treatment protocol. We aim to describe the results of this comparative cohort study between Group 1 of the prospective case series (n=50) and Group 2 of the retrospectively obtained cohort group (n=50). This article mainly highlights the technical consideration of the DISH10 maneuver and elaborates the probable mechanisms by which it provides quick symptomatic relief and reduces the treatment duration.
Methods
This comparative cohort study included 100 admitted patients who developed PDPH following neuraxial anesthesia for lower extremity orthopedic surgeries performed at a tertiary care centre (Ganga Medical Centre and Hospitals Private Limited, Coimbatore, India) from January 2018 to March 2021. The study was divided into two groups: Group 1 included a prospective case series of 50 cases in which PDPH was treated with DISH10 maneuver, and Group 2 included a retrospective cohort of 50 cases in which PDPH was treated with conventional conservative management with or without SPGB.
Inclusion criteria
1. Patients (ASA grade I and II) of all age groups admitted for lower extremity orthopedic surgeries under neuraxial blockade and diagnosed to have PDPH in the postoperative period
2. PDPH patient treated with DISH10 maneuver (Group 1)
3. PDPH patients treated with conventional conservative management with or without sphenopalatine ganglion block (Group 2).
Exclusion criteria
1. ASA III/IV patients
2. Patient’s refusal (Group 1)
3. Patients with PDPH treated with DISH10 maneuver after the failure of conservative trial for more than two days
4. Incomplete case related documents (Group 2)
5. Polytrauma patients
6. Patients who are defaulters.
Group 1
After obtaining approval from the Institutional Review Board (IRB) committee at Ganga Medical Centre & Hospitals Private Limited, a total of 50 patients satisfying the inclusion criteria were included in this cohort of prospective case series. In these patients, the PDPH was diagnosed by the duty anesthetist, based on the following criteria
I. Positional headache: Aggravated in sitting/erect position and improved in supine/lying position
II. Location: Frontal or occipital, or both
III. Associated symptoms: Generalized symptoms (nausea, dizziness, neck stiffness) or localized symptoms (auditory and/or visual hallucinations/phenomena)
IV. Onset: Within 12–48 hours to rarely more than five days post neuraxial blockade.
The severity of the headaches was graded based on the visual analog scale (VAS) from 0-10 (where 0 =no pain and 10 =the worse pain imaginable) and the functional grading (FG) from 1-3 scale (where, 1= headache not interfering with regular daily activity, 2= headache requiring periodical bedrest to get relief, and 3= severe headache not allowing the patient to sit up and eat).
Combining both scores, the severity of postdural puncture headache graded as grade 0- no headaches, 1- mild (corresponds to FG 1+ and VAS 1-3), 2- moderate (corresponds to FG 2+ and VAS 4-7), and 3- severe (corresponds to FG 3+ and VAS 8-10).
We kept all patients under observation in the special ward with standard basic hemodynamic monitoring facilities to record events, provide further care, and assist them in performing the DISH10 maneuver. We advised them to increase fluid intake, take bed rest, and continue their ongoing multimodal analgesics (oral paracetamol 1g qid, aceclofenac 100 mg bd, and pregabalin 75 mg hs). We explained the steps of the DISH10 maneuver and its possible side effects like dizziness, nausea, or vomiting (Table 1). After discussing the risk-benefit ratio, we asked them to perform the DISH10 maneuver at regular intervals.
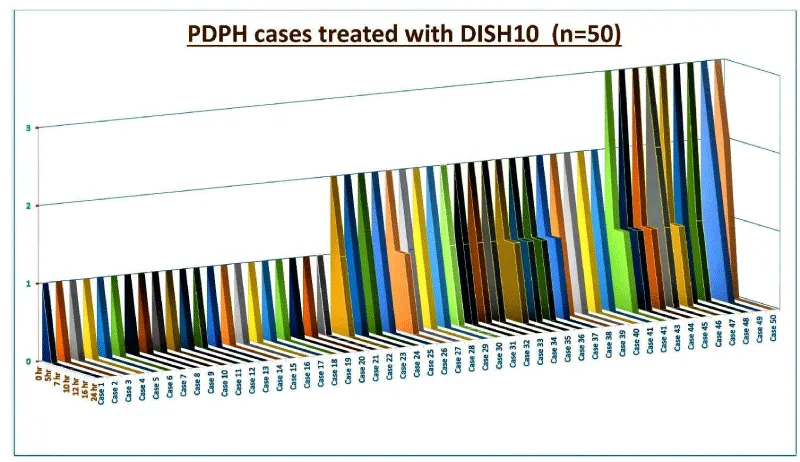
We noted the severity of the headache after the DISH10 maneuver at the 5th, 7th, 10th, 12th, 18th, and 24th hours (Figure 1). The first ten patients were kept under observation in a high dependency unit for 24 hours, 20 patients for 12 hours, and the remaining 20 patients for 8 hours only. We shifted all the patients to their rooms/wards from the monitoring facility after confirming adequate relief of the symptoms. We advised them to inform the duty anesthetist about the symptoms’ recurrence and perform the same maneuver for 2-3 hours. During hospital discharge, we advised all patients to contact the attending anesthetist (one of the authors) upon the recurrence of symptoms and perform the same maneuver as advised for another 2-3 hours at that time. Fortunately, no patients reported the relapse of headaches during telephonic as well as physical follow-ups. All patients included in this prospective case series, or their next-of-kin provided informed consent for anonymous data recording and sharing concerning this procedure.
Group 2
We performed the retrospective analysis of the case-related documents (between the year 2018-2021) of the PDPH patients who received only conservative management (with or without SPGB). A total of 50 cases were included (as per the inclusion criteria) in this retrospective cohort group, divided into two subgroups. The first subgroup (n=33) consists of those PDPH patients who received only conservative management such as bed rest, adequate hydration, multimodal analgesics, and caffeinated drinks. The second subgroup (n=17) consists of patients who received SPGB three times a day with conservative management.
Outcome measurement
The patient’s demographics, type of neuraxial anesthesia, size/type of spinal needle, and the onset and severity of the PDPH symptoms were noted (Table 2). The outcomes were measured about the effect of spinal needle size/type to develop PDPH, time to develop PDPH (headache freedom time), treatment duration or time to outcome (time to become symptoms-free), and the effect of headache severity on time to outcome.
Statistical analysis
Data were coded and recorded in the MS Excel spreadsheet program and statistically analyzed (Table 3) using IBM SPSS statistics (Statistical Package for Social Sciences, software version 23.0, IBM Corp., Chicago, USA). Descriptive statistics were elaborated in means/standard deviations and medians/IQRs for continuous variables, frequencies, and percentages for categorical variables. Data were presented graphically (Figures 2-4) wherever appropriate for data visualization using histograms/box-and-whisker plots/column charts for continuous data and bar charts/pie charts for categorical data. When comparing two groups, the independent sample ‘t’ test was used for continuously distributed data. For non-normally distributed data, appropriate nonparametric tests like the Wilcoxon Test were used. A chi-squared test was used for group comparisons for categorical data. Fisher’s Exact Test was used if the expected frequency in the contingency tables was found to be <5 for >25% of the cells. Linear correlation between two continuous variables was explored using Pearson’s correlation (if the data were normally distributed) and Spearman’s correlation (for non-normally distributed data). Statistical significance was kept at p < 0.05.
Results
Out of 117 patients included in this comparative cohort study, 57 patients were enrolled in Group 1 (prospective case series) and 60 patients in Group 2 (retrospective cohort). Out of 57 patients in Group 1 (DISH10 group), seven patients were excluded due to failure to complete steps as per instructions (4 patients) and delay in treatment due to nighttime (3 patients). Out of 60 patients in Group 2 (conservative group), ten patients were excluded due to incomplete and misleading data in their case sheets. In this comparative cohort study,
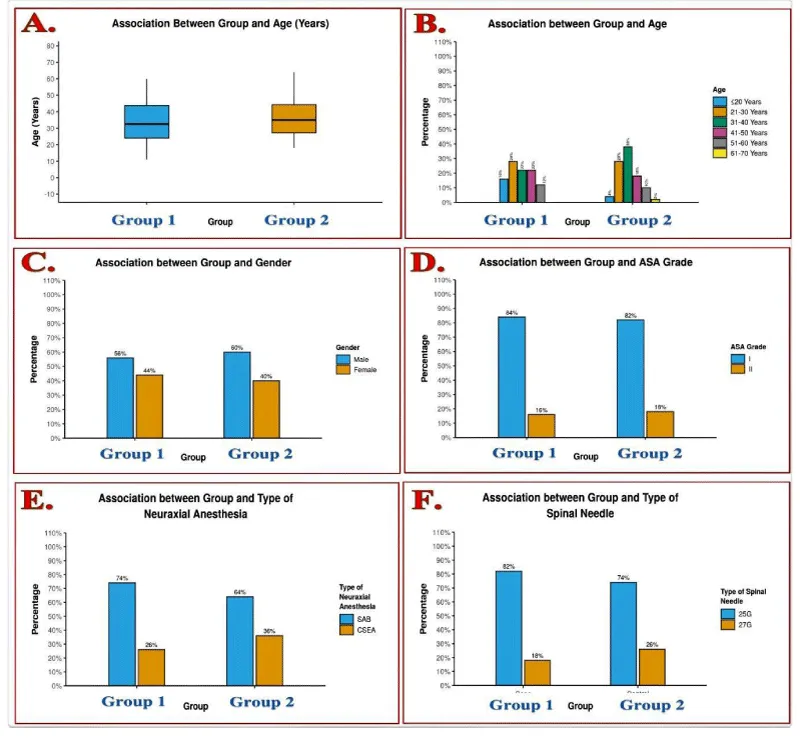
• The most common age of presentation of PDPH was in the age group of 11-64 years. The median (IQR) of age (years) in the Group 1 was 32.5 (24-43.75), and Group 2 was 35 (27.25-44.25).
• 58% of the PDPH patients were males, whereas 42% were females.
• 83 (83%) patients (42 in Group 1 and 41 in Group 2) were of ASA I grade, and 17 (17%) patients (8 in Group 1 and 9 in Group 2) of ASA II grade.
• 69 (69%) patients (37 in Group 1 and 32 in Group 2) received SAB, whereas 31(31%) patients (13 in Group 1 and 18 in Group 2) received CSEA.
• The spinal needle used in 78 (78%) patients (41 in Group 1 and 37 in Group 2) was 25G of Quincke type, and in 22 (22%) patients (9 in Group 1 and 13 in Group 2) was 27 G of Whitacre type.
• The headache severity of 36 (36%) patients (21 in Group 1 and 15 in Group 2) was mild, 43 (43%) patients (20 in Group 1 and 23 in Group 2) was moderate, and 21 (21%) patients (9 Group 1 and 12 in Group 2) was severe.
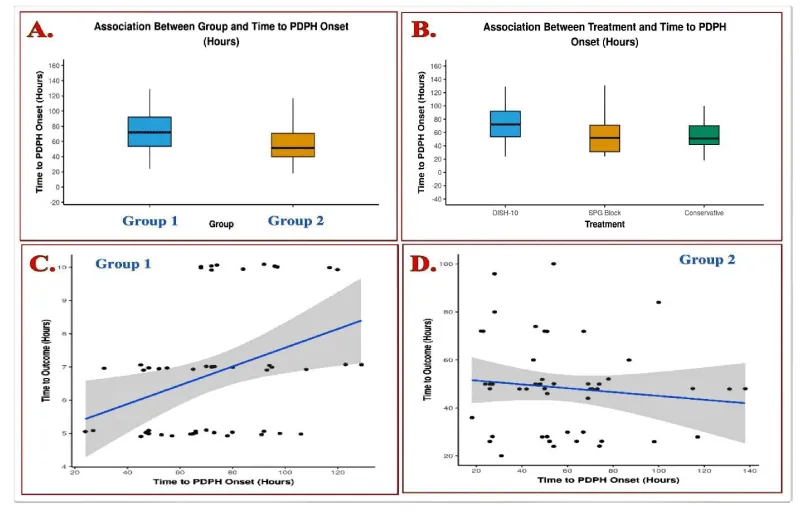
• The time to PDPH onset or headache freedom time (hours) in Group 1 ranged from 24– 129, and Group 2 ranged from 18 - 138. The median (IQR) of time to PDPH onset (hours) in the Group 1 was 72 (53.5-92), and Group 2 was 51.5 (39.75-70.75).
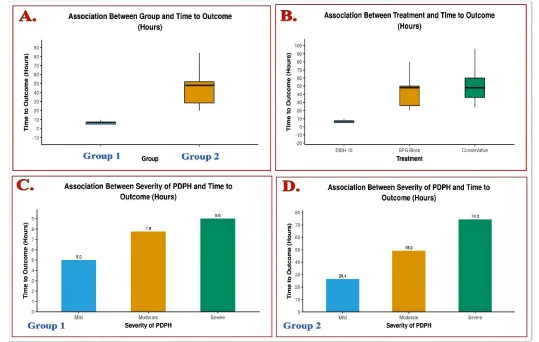
• The time to outcome (hours) in Group 1 ranged from 5–10, and in Group 2 ranged from 20-100. The median (IQR) of time to outcome (hours) in the Group 1 was 7 (5-7), and Group 2 was 48 (28.5-52).
• The time to outcome (time required to make patient symptom-free/treatment duration) in the patients with severe grade headache was more than others.
Discussion
The findings of our comparative cohort study support our hypothesis and suggest that PDPH patients treated with the DISH10 maneuver will experience quick symptomatic relief due to faster spontaneous recovery. The cohort of prospective case series (Group 1) demonstrates an alternative to invasive procedures for PDPH with a high success rate within 24 hours of therapy. Comparing this group with the retrospective cohort (Group 2) evinced reduced time to outcome (treatment duration), making patients ready to discharge. Other findings like the occurrence of PDPH commonly in the younger population (median age in years of Group 1 is 32.5, and Group 2 is 35) and its association with large-bore needles (78% with 25 G Quincke-type and 22% with 27 G Whitacre-type) are consistent with available literature of PDPH [7-13].
The DISH10 maneuver is self-controlled (without the requirement of any extra person), non-invasive (without the need of any invasive procedure or specialized instrument or drug), and a cost-effective (early discharge and less hospital stay) option for PDPH management. However, this intervention involves a specialized maneuver that helps in increasing intrathoracic and intraabdominal pressure at high-frequency intervals. The rationale for a favorable outcome with this technique is multifactorial. The knowledge of PDPH etiopathogenesis is essential to understand the mechanism of the DISH10 maneuver.
The etiopathogenesis of PDPH includes CSF leakage from the subarachnoid space (through the meningeal puncture), resulting in disruption of CSF homeostasis due to a decrease in CSF volume and pressure [14]. The CSF is produced primarily in the choroid plexus at a rate of approximately 0.20-0.35 mL/min (around 25 ml/hr or 500 ml/day) and reabsorbed through the arachnoid villi [15]. At any moment, the total CSF volume in adults is maintained at around 125-150 mL, of which approximately half is extracranial [16]. Loss of approximately 10% of total CSF volume predictably results in the development of typical PDPH symptoms, which resolve promptly with the reconstitution of this deficit [17]. Concurrent intracranial hypotension due to persistent CSF leak may lead to adenosine-mediated cerebral and meningeal vasodilation, which may cause or contribute to the headache [18]. Thus, the headache following CSF hypotension develops due to a bimodal mechanism involving both loss of intracranial support (buoyant support) and reciprocal cerebral vasodilation (predominantly venous) [13] Diminished buoyant support results in radiologically demonstrable ‘sagging’ of intracranial structures mainly in the upright position, resulting in traction and pressure on pain-sensitive structures (dura, cranial nerves, bridging veins, and venous sinuses) within the cranium [13]. The stretching of various neural elements causes typical symptoms of PDPH like positional headache, nuchal pain, and other associated auditory/visual/vestibular symptoms (Table 4).
The anatomical and physiological factors of the neuraxis affect the closure of dural holes and CSF leakage. The epidural space is considered a potential space with negative pressure ranging from -1 cm H2O (lumbar region) to -10 cm H2O (thoracic region). The measured CSF pressure via lumbar puncture is 10-18 cm H2O (8-15 mmHg or 1.1-2 kPa) in the lateral position and 20-30 cm H2O (16–24 mmHg or 2.1–3.2 kPa) in the sitting position [19]. The negative epidural pressure and the positive subarachnoid pressure create a high-pressure gradient across the dural opening, resulting in a continuous suctioning effect leading to persistent CSF drainage. This vacuum effect may impede the spontaneous closure of the dural hole and cause a massive CSF collection outside the dura.
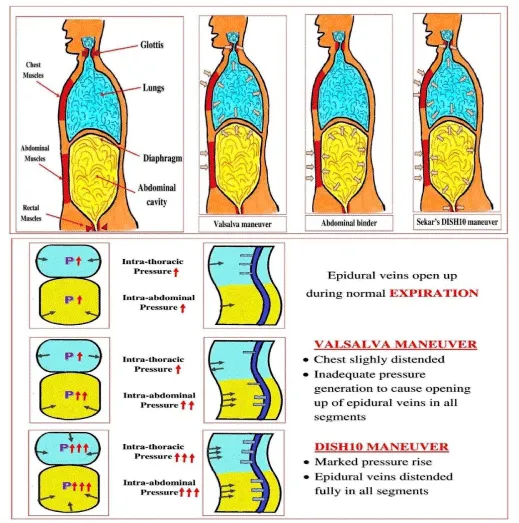
Factors modifying negative epidural pressure include the head-up position, cough, Valsalva, Queckendsted, and abdominal wall compression maneuvers [20]. Moreover, at the lumbar level, the 30% head-up position decreases the epidural pressure to half that at the supine position [21]. The Valsalva maneuver (VM) and cough cause increased epidural pressure in the cervical, thoracic, and lumbar areas. The abdominal compression binder mainly increases transabdominal pressure. The VM is one of the vagal maneuvers described mainly for restoring heart rhythm, diagnosing autonomic disorders, and treating clogged ears. There is a subtle difference between VM and DISH10 maneuver. The VM is a “forced expiration against a closed glottis,” leading to a decrease in thoracic cavity volume at the end of expiration, which causes ineffective pressure generation to increase the epidural blood flow. In contrast, the DISH10 is a “deep inspiratory hold” combined with squeezing (chest and abdomen), increasing intrathoracic and intra-abdominal pressure simultaneously. These changes in the visceral cavity pressures can be transmitted to the epidural space directly, generating and maintaining sufficient pressure to engorge the epidural venous plexus (Figure 5).
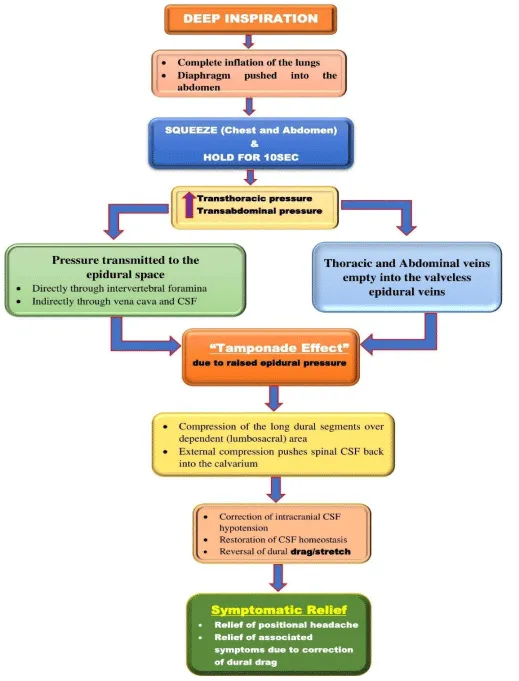
So, the DISH10 maneuver is based on the principle of generating adequate transthoracic and transabdominal pressure to be effective in increasing the blood flow in the epidural venous plexuses (Figure 6). The resulting engorgement of the epidural veins produces an external “tamponade effect” that may compress long dural segments, mainly in the lumbosacral region (dependent area). Compression of the dural segment pushes the CSF back into the calvarium, correcting the intracranial CSF hypotension. This tamponade effect also reverses the pressure gradient across the dura, stopping the CSF leakage. We believe that an epidural blood patch also creates a similar tamponade effect over the lumbosacral region resulting in a similar effect (pushing the CSF back into the calvarium), causing quick relief from the symptoms.
Maintaining the positive epidural pressure for a significant duration reverses dural drag/stretch, allows new CSF formation to replace lost volume without any further leakages, and restores CSF homeostasis. The epidural pressure peaks up to +60 cm H2O after the epidural saline injection and fell exponentially to zero within 10 minutes [22]. Thus, to maintain continuous epidural pressure, we kept the DISH10 maneuver frequency as ten cycles per hour, keeping a gap of at least six minutes between them. This continuous external pressure on the dura may also keep ends of the dural hole approximated and align the arachnoid stretch, potentially leading to complete closure of the meningeal defect that allows it to heal spontaneously.
The ideal non-invasive techniques should serve three purposes. Firstly, it should reverse the pressure gradient across the dural hole to stop CSF leakage by creating positive pressure in the negative epidural space. Secondly, it should correct the intracranial CSF hypotension by pushing the CSF back into the calvarium. Thirdly, it should immediately improve PDPH symptoms by correcting the brain sagging and dural stretching/dragging. Thus, the DISH10 maneuver fulfills all three essential purposes making it the most effective adjunct to the conservative treatment.
Transient increase in the transthoracic and transabdominal pressure in this maneuver may cause hemodynamic fluctuations (Table 1). A significant decrease in the cerebral perfusion pressure (CPP) has been demonstrated during the strain phase [23] due to an abrupt reduction in mean arterial pressure (MAP). It can result in syncope due to an acute reduction in cerebral oxygenation leading to unconsciousness. For this reason, we believe in avoiding this maneuver in patients with contraindications to traditional vagal maneuvers or in patients who develop presyncope with this maneuver. Fortunately, such complications were not seen in all our patients. The patient’s position has a modest effect, with a more considerable decrease in BP during the squeezing phase with the patient seated or standing (and a greater risk of syncope) [24]. As a precaution, we recommend doing this maneuver in a lying-down position with raised (15-20 degree) foot end of the bed to maintain venous return and avoid syncopal attacks. The patient should not be allowed to sit before 5 hours of treatment.
The possibility of hemodynamic fluctuation necessitates vigilant monitoring (of vital parameters) in patients with cardiac issues (like ischemic heart disease or fixed cardiac output states) during the performance of DISH10. However, we found it safe and effective in such populations without any complications. The patients with a high body mass index (i.e., obesity) may have a decreased risk of developing PDPH, possibly secondary to a beneficial effect of increased intra-abdominal pressure [25]. The clinicians should be well conversant with its applied physiology and use it judiciously to avoid associated complications.
Most of the treatments of PDPH mainly focus on either stoppage of CSF leakage or correcting intracranial CSF hypotension. These treatments include oral/intravenous fluids and analgesics, caffeine, 5-HT receptor antagonist, and most importantly, the epidural blood patch (EBP). The EBP technique, though considered a gold standard treatment, is an invasive procedure and not free of side effects. It is presumed to act by sealing the dural hole and stopping CSF leakage. Bed rest is also an essential component of conservative management, as lying down position shifts CSF back into calvarium, correcting CSF hypotension and improving symptoms. Due to the paucity of quality literature, many questions regarding treatment options of PDPH are still unanswered. However, spontaneous recovery is possible in PDPH patients without any treatment in 4-5 days. In 70%, the headache resolves in a week, and in 87% of cases, it resolves within six months [26] or longer than six months [27]. Severe PDPH can cause delayed recovery and discharge from the hospital, which adds to the treatment cost. The patients in Group 1 (case series) had improvement in symptoms within 24 hours of starting the maneuver, which further avoided a delay in discharge and thus reduced total hospital stay. Thus, by causing faster relief of PDPH symptoms through a probable sealant effect and tamponade effect described before, the DISH10 maneuver aids spontaneous recovery.
The limitation of our study is its design and limited sample size. We did not include other subgroups of patients like pregnant ladies or children in this study. We have not evaluated the efficacy of DISH10 in patients with PDPH following diagnostic lumbar puncture or myelography. Moreover, a prospective randomized case-control trial would have produced a better level of evidence and lesser bias.
Conclusions
PDPH has become a rare complication due to advances in anesthesia techniques, improved knowledge of pathophysiology, and the implementation of preventable measures. Diagnosis and treatment options of PDPH depend on its presentation as well as the severity of symptoms. Spontaneous recovery from the PDPH is directly proportional to the severity of the symptoms.
The non-invasive DISH10 maneuver promotes enhanced recovery from the PDPH. It may be a safer alternative in the PDPH management over the other invasive interventions due to its better side-effect profile, noninvasiveness, and cost-effectiveness. However, well-designed prospective comparative clinical investigations with a larger sample size are warranted to validate these findings, beliefs and speculations.
We would like to take this opportunity to express gratitude to all clinical staff at Ganga Medical Centre and Hospital Private Limited.
Contributorship Statement
K.S: Involved in the conceptualization of the technique developed by CS. Coined term Sekar’s DISH10 maneuver. Involved in implementing treatment protocol in cases included. Involved in reference collection and sorting work with the help of HD and TM. Designed manuscript content and co-wrote the paper. Took the lead in manuscript writing and illustration compilation.
C.S: Major contributor in developing and innovating DISH10 maneuver. Provided scientific guidance for manuscript writing as well as essential content for the conceptualization of the idea. Guided the content of the manuscript and co-wrote the paper. The approved final version of the manuscript.
H.D: Involved in the anatomical description and information collection required for the manuscript writing. Co-wrote and proofread the manuscript.
T.M: Provided clinical data required for the manuscript writing. Co-wrote and proofread the manuscript.
All authors provided critical feedback and helped shape the manuscript.
- Bier A (1899) Versuche ueber cocainisirung des rueckenmarkes. Dtsch Zeitschr Chir 51: 361-369. Link: https://bit.ly/36874mC
- Alam M, Raheen M, Iqbal K, Chowdhury M (2011) Headache following Spinal Anaesthesia: A Review on Recent Update. Journal of Bangladesh College of Physicians and Surgeons 29: 32-40. Link: https://bit.ly/3w3HpWU
- Reina MA, López A, van Zundert A, De Andrés JA (2015) Ultrastructure of dural lesions produced in lumbar punctures. In: Reina MA. Atlas of functional anatomy of regional anesthesia and pain medicine. New York: Springer 767–793. Link: https://bit.ly/2Tp6RJ5
- De Haan JB, Chrisman OM, Lee L, Ge M, Hernandez N (2019) T4 Erector Spinae Plane Block Relieves Postdural Puncture Headache: A Case Report. Cureus 11: e6237. Link: https://bit.ly/3Aht1ha
- Niraj G, Kelkar A, Girotra V (2014) Greater occipital nerve block for postdural puncture headache (PDPH): a prospective audit of a modified guideline for the management of PDPH and review of the literature. Journal of clinical anesthesia 26: 539–544. https://doi.org/10.1016/j.jclinane.2014.03.006
- Nair AS, Rayani BK (2017) Sphenopalatine ganglion block for relieving postdural puncture headache: technique and mechanism of action of block with a narrative review of efficacy. Korean Journal of pain 30: 93–97. https://bit.ly/3qFUpRC
- Kwak KH (2017) Postdural puncture headache. Korean Journal Anesthesiology 70: 136–143. Link: https://bit.ly/36js4ap
- Kuczkowski KM (2004) Post-dural puncture headache in the obstetric patient: an old problem. New solutions. Minerva Anestesiol 70: 823–830. Link: https://bit.ly/3dsqPJR
- Khraise WN, Allouh MZ, El-Radaideh KM, Said RS, Al-Rusan AM (2017) Assessment of risk factors for postdural puncture headache in women undergoing cesarean delivery in Jordan: a retrospective analytical study. Local and Regional Anesthesia 10: 9-13. Link: https://bit.ly/3dw9nnR
- Weji BG, Obsa MS, Melese KG, Azeze GA (2020) Incidence and risk factors of postdural puncture headache: prospective cohort study design. Perioperative Medicine 9: 32. Link: https://bit.ly/3dwBiE8
- Tarekegn F, Eshetie S, Aregawi A, Moges K (2017) Assessment of the Prevalence and Associated Risk Factors of Post Dural Puncture Headache (PDPH) after Cesarean Section Delivery under Spinal Anesthesia. J Anesth Crit Care Open Access 8: 00330. Link: https://bit.ly/2UjmmCj
- Wang F (2014) Post Dural Puncture Headache – We Can Prevent It. Pain and Treatment. Intech Open. Link: https://bit.ly/3dxhnF1
- Hadzic A (2017) Hadzic’s textbook of regional anesthesia and acute pain management, 2e. Link: https://bit.ly/3jyNd8i
- Grände PO (2005) Mechanisms behind postspinal headache and brain stem compression following lumbar dural puncture--a physiological approach. Acta Anaesthesiologica Scandinavica 49: 619-626. Link: https://bit.ly/3x90w3e
- Chan M, Amin‐Hanjani S (2010) Cerebrospinal Fluid and its Abnormalities. eLS. Link: https://bit.ly/2UivYx7
- Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. European Annals of Otorhinolaryngology, Head and Neck Diseases 128: 309–316. Link: https://bit.ly/3xbxm3d
- Kunkle EC, Ray BS, Wolff HG (1943) Experimental studies on headache: analysis of the headache associated with changes in intracranial pressure. Arch NeurPsych 49: 323-358. Link: https://bit.ly/3jw8cbG
- Boezaart AP (2001) Effects of cerebrospinal fluid loss and epidural blood patch on cerebral blood flow in swine. Regional Anesthesia and Pain Medicine 26: 401–406. Link: https://bit.ly/3AkeQYm
- Agamanolis D (2011)“Chapter 14 – Cerebrospinal Fluid: THE NORMAL CSF”. Neuropathology. Northeast Ohio Medical University.
- Usubiaga JE, Moya F, Usubiaga LE (1967) Effect of thoracic and abdominal pressure changes on the epidural space pressure. Brit J Anaesth 39: 612-618. Link: https://bit.ly/36c9lgx
- Iwama H, Ohmori S (2000) Continuous monitoring of lower thoracic epidural pressure. Journal Critical Care 15: 60–63. Link: https://bit.ly/3xbvsj9
- Usubiaga JE, Moya F, Usubiaga LE (1967) A note on the recording of epidural negative pressure. Canadian Anaesthetists’ Society Journal 14: 119–122. Link: https://bit.ly/2SCKijG
- Wendling W, Sadel S, Jimenez D, Rosenwasser R, Buchheit W (1994) Cardiovascular and cerebrovascular effects of the applied Valsalva manoeuvre in anaesthetized neurosurgical patients. Eur J Anaesthesiol 11: 81–87. Link: https://bit.ly/3xndwCx
- Marc BD, Nobutaka H, Uwe W (2009) Encyclopedia of Neuroscience. Springer.
- Faure E, Moreno R, Thisted R (1994) Incidence of postdural puncture headache in morbidly obese parturients. Reg Anesth 19: 361-363. Link: https://bit.ly/3w3SMhu
- Vandam LD, Dripps RD (1956) Long-term follow-up of patients who received 10,098 spinal anesthetics; syndrome of decreased intracranial pressure (headache and ocular and auditory difficulties). Journal of the American Medical Association 161: 586-591. https://bit.ly/3hjBxDw
- Klepstad P (1999) Relief of postural post dural puncture headache by an epidural blood patch 12 months after dural puncture. Acta Anaesthesiologica Scandinavica 43: 964– 966. Link: https://bit.ly/3xbzLLx
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
