Archives of Anatomy and Physiology
Dental pathologies in the nigerian local pigs (Sus scrofa)
1Department of Veterinary Anatomy, University of Ibadan, Nigeria
2Department of Anatomy, College of Medicine, University of Ibadan, Nigeria
3Department of Veterinary Medicine, University of Ibadan, Nigeria
4Department of Veterinary Anatomy, Federal University of Agriculture, Abeokuta, Nigeria
Author and article information
Cite this as
Okandeji ME, Femi-Akinlosotu OM, Omotosho OO, Olopade JO (2022) Dental pathologies in the nigerian local pigs (Sus scrofa). Arch Anat Physiol. 2022; 7(1): 001-008. Available from: 10.17352/aap.000019
Copyright License
© 2022 Okandeji ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Dental pathologies are noticeable alterations or deviations from normal dental architecture and can be influenced by genetic or environmental factors. This present study aimed to identify and report the dental pathologies in the Nigerian local pig.
Materials and methods: The cleaned skulls of 47 local pigs, aged between 3-51 months were assessed for observable dental abnormalities such as missing teeth, fractured teeth, persistent deciduous teeth, dental caries, dental calculus, and tooth rotation.
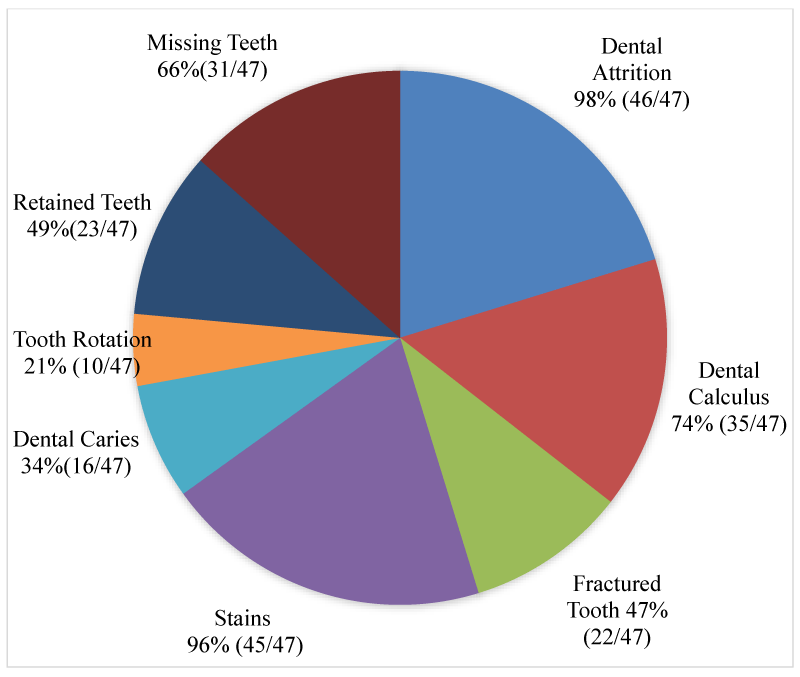
Results: The study revealed that 98% of the skulls had dental attrition while 96% had stained teeth. About 66% had a least a missing tooth while 78% of adult skulls had at least one persistent deciduous tooth. Fractured tooth, dental calculus, dental caries, and tooth rotation were observed in 46.8%, 74.4%, 34% and 21.3%, respectively. The most common persistent deciduous tooth was the second maxillary incisor, whereas the most commonly missed tooth was the first mandibular premolar, which was bilateral in 75% of affected skulls. Dental calculus was not observed on skulls below 6 months, while the incisor tooth was the most affected tooth by dental attrition.
Conclusions: The Nigerian local pig, like other breeds, is susceptible to and has dental pathologies. The data obtained from this study will be beneficial to farmers, as early detection of dental abnormalities will promote productivity and reduces economic losses in pig husbandry. It will also be useful to researchers, especially those using pigs in Nigeria as a model for translation research and comparative dental studies.
NLP: Nigerian Local pig; UI-ACUREC: the University of Ibadan-Animal Care and Use of Research Ethics Committee; Spp: Species
Introduction
Teeth are hard, whitish structures in the oral cavities of vertebrates and represent the modification of bony dermal plates found in ancestral fish [1,2]. They are made up of tissues of varying density and hardness, including enamel, dentin, and cementum, which surround a pulp cavity containing neurovascular bundles [3,4]. Mammalian teeth are important masticatory organs. In higher animals, they participate in phonation and the protection of support organs. Species variations however exist, depending on the type of food each species feeds on [5,6].
Dental pathologies are described as noticeable alterations or deviations from normal dental architecture, which occur during the development of dental structures. In animals, they have been attributed to domestication, as certain anomalies seem to present higher frequencies in domestic species than in their wild counterparts because of artificial selection, inbreeding, and general shortening of the skull [7-11].
Developmental disorders can be genetically or environmentally induced, resulting in alterations in the number, size, shape, or structure of affected teeth [12]. However, it may be difficult to ascertain what is responsible for specific anomalies, as it is believed that these conditions develop over a period of time [7]. Congenital disorders like polyodontia, hypodontia, and malocclusion can cause abnormal attrition, leading to dental diseases like caries and periodontitis, due to the impaction of fibers between teeth [13]. These diseases have been associated with disease-causing bacteria.
Pigs are omnivores, possessing diphyodont, heterodont, and brachydont dentition, except the male canine teeth (tusks), which are aradicular, hypsodont, and elodont in nature [14]. The porcine deciduous dental formula is 2(i 3/3, c 1/1, p 3/3), while the permanent dental formula is 2(I 3/3, C 1/1, P 4/4, M 3/3) [14-16].
Pigs are regarded as suitable research models in biomedical and pharmacological studies due to their morpho-physiological similarities with humans, and the ease of accessing disease models [14,17-18]. Pig models, which are often genetically-engineered breeds of pigs and can mimic human disease conditions, have been used to investigate regenerative processes following periodontal stem cell application [19], dental implants [20], and regrowth of teeth and jawbones [21]. In addition, pigs have diphyodont dentition, making them excellent models for studying teeth replacement [22].
The Nigerian local pigs (NLP) or the West African Dwarf pigs are considered to be domesticated indigenous pigs because they are usually found living within communities inhabited by humans, raised semi-intensively. They are small-bodied pigs with short foreheads, straight tails, elongated snouts, and medium-sized, semi-erect ears. They have narrow body conformation with relatively long legs and slightly inclined rumps. They have predominantly black coats, with others being brown, white, and black or patchy and spotted [23,24]
Although several studies have attempted to describe dental pathologies in the wild and domestic pigs [7-10,13,14,25], there appears to be a dearth of information on the dental pathologies in the NLP. This study aimed to record and report the prevalence of dental pathologies in the NLP (Sus scrofa), in an ongoing effort to provide baseline information on this breed of pigs.
Materials and methods
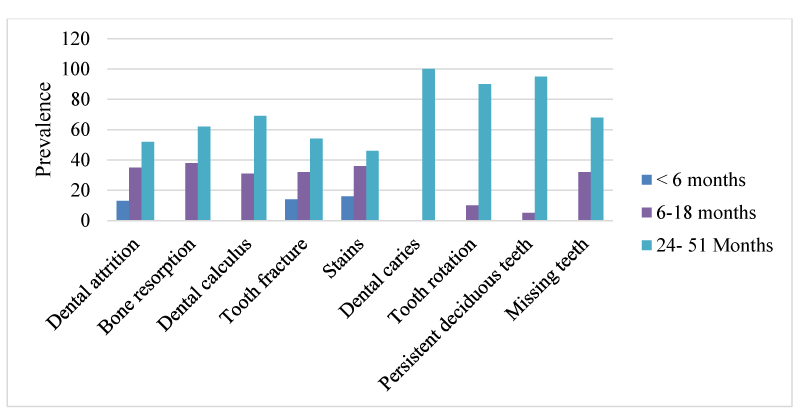
Forty-seven pig heads (21 males, 26 females) were obtained based on parameters of apparent good health and absence of cranial deformities. The pigs were used in a previous study (ethical approval: UI-ACUREC/20/009). They were grouped by sex and age (below 6 months, 6-15 months, and 24-51 months). Pigs below 6 months of age had predominantly deciduous dentition, those between 6 and 15 months were characterized by the presence of both deciduous and permanent dentition, a condition called “mixed dentition”, while pigs between 24 and 51 months were regarded as adults, having predominantly, permanent dentition. The age of each animal was obtained from available birth records and varied between 3 and 51 months. The severed heads were prepared using a modification of the hot water maceration technique described by [26] and the mandibles separated from the skull (cranium) so that a complete view of the dental profile could be assessed. A systematic assessment of the dental abnormalities in the upper and lower jaws was done. The teeth were examined in detail and the lesions present were recorded. Pictures of the skulls were taken with a Nikon COOLPIX L320® (16.1 Megapixels) digital camera. Prevalence was calculated as the percentage occurrence of an abnormality per animal amongst the total number of animals observed. All observed dental pathologies were recorded and presented.
Results
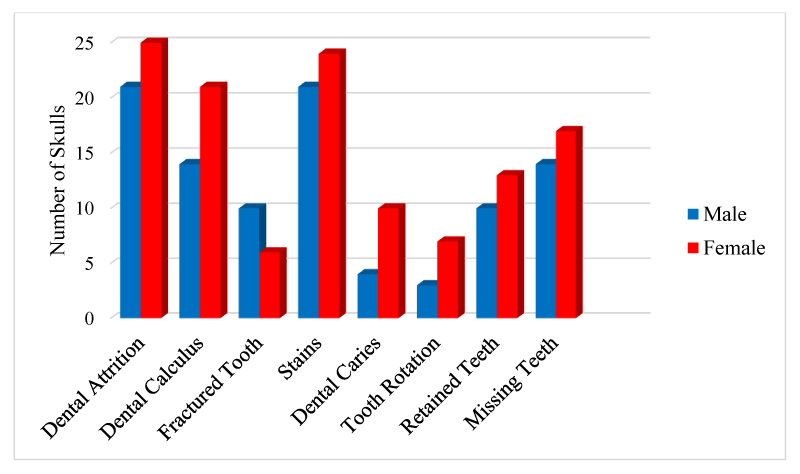
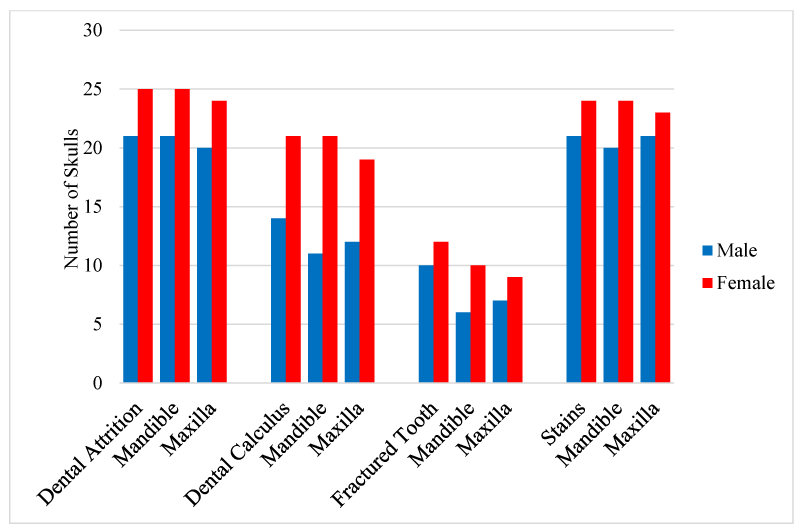
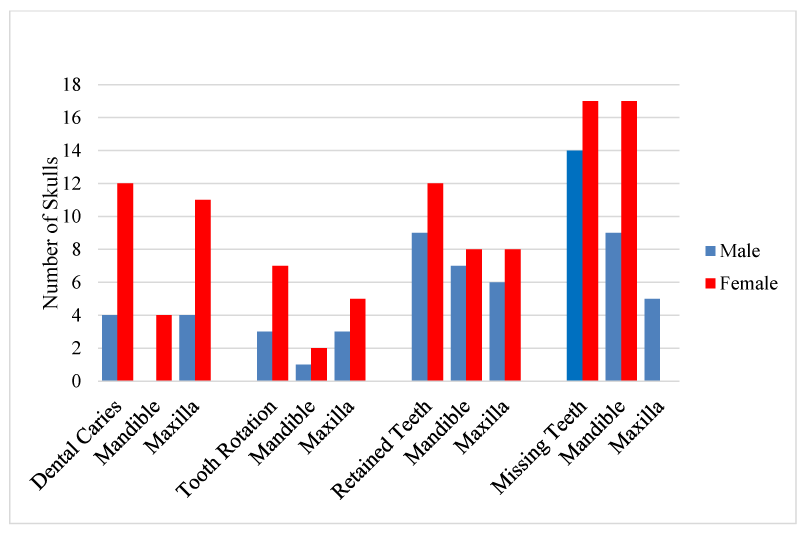
The results showed that dental pathologies were present in all the examined skulls, with at least one type of dental pathology being present in each skull. All observed dental pathologies were presented in Table 1, prevalence and sex distribution of mandibular and maxillary dental abnormalities were presented in charts (Figures 1-5) while pictorial evidence was stated in Figures 6-11.
Dental attrition
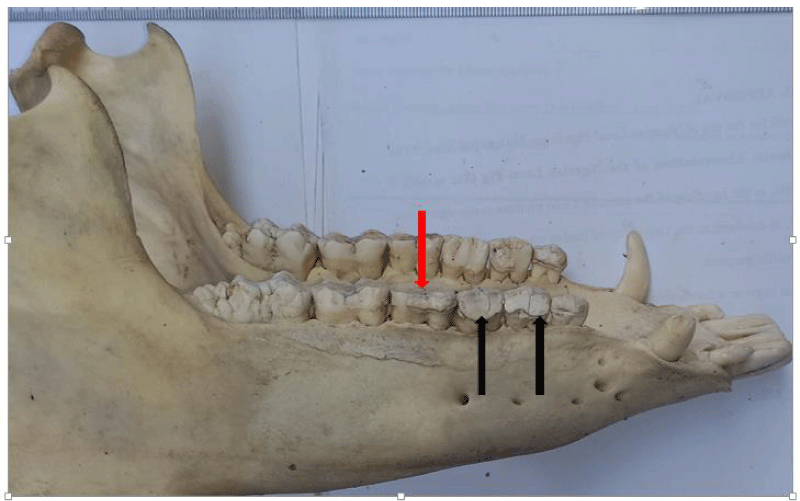
Overall, attrition was present in 97.9% (46/47) of examined skulls. Attrition was observed in 100% (46/46) of the mandibles and 95.7% (44/46) of the maxillae. It was observed in 13% (6/46) of skulls less than 6 months, 34.8% (16/46) of skulls between 6-18 months, and 52.2% (24/46) of skulls between 24-51 months. A total of 267 teeth showed signs of attrition: incisor teeth (84), canine teeth (56), premolar teeth (72), and molar teeth (55), with the premolar teeth and the first molar tooth showing more severe lesions.
Dental calculus
Calculus was observed in the teeth of 74.4% (35/47) of the examined skull but was not apparent in the teeth of the skulls of pigs less than 6 months of age. The incidence of the calculus was higher in females (60%, 21/35), than males (40%, 14/35) and higher in the mandible (91%), compared to the maxillae (89%). Moderate and severe cases of calculus were observed in adult skulls (>27 months), with the premolar teeth being mostly affected. These tightly adhering hard deposits were found on the crowns of the teeth, coronal to the gingiva margins.
Fractured tooth
Tooth fracture was observed in 46.8% (22/47) of the examined skulls (12 females, 10 males). Fractured teeth were observed on the mandibles and maxillae (n=16, respectively). A total of 77 teeth had evidence of fracture, across all age groups (19 deciduous, 58 permanent). Teeth affected included incisor teeth (6), canine teeth (14), premolar teeth (42), and molar teeth (6).
Stains
Stains were evident in 95.7% (45/47) of the skulls examined (24 females and 21 males). Two adult female skulls did not show any observable sign of tooth staining. All categories of teeth showed varying degrees of staining across all age groups.
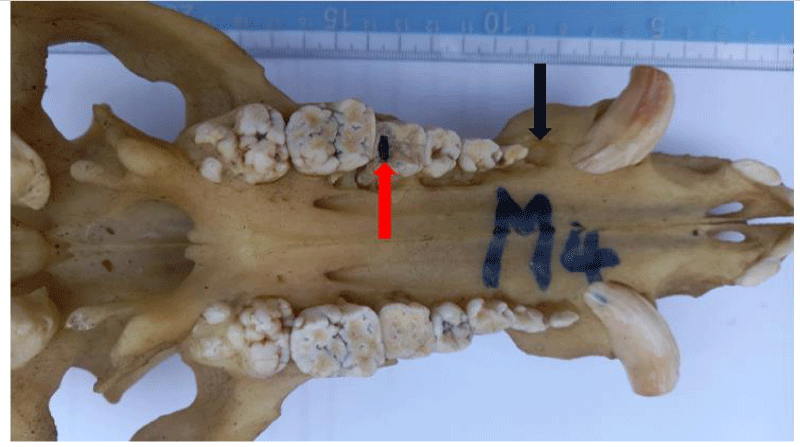
Dental caries
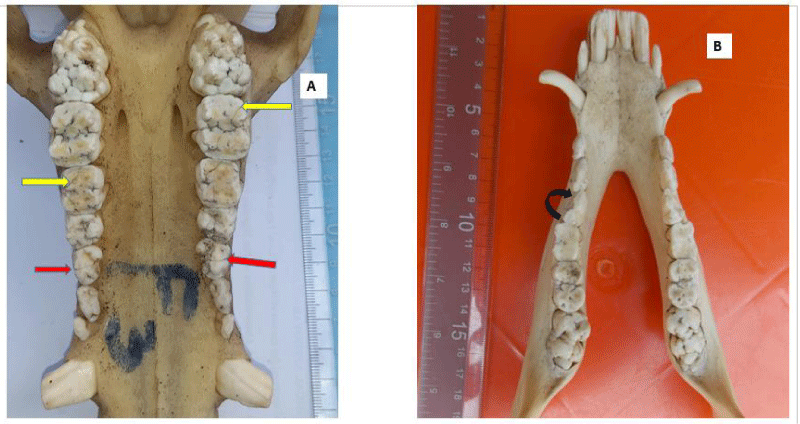
Dental caries were observed in 34% of the skulls (16/47), affecting 34 teeth in total. The fourth premolar was the most dominant tooth type affected by caries (n=24), followed by the third premolar (n=4), first molar (n=3), second premolar (n=2), and the first premolar tooth (n=1) respectively. Dental caries were observed in only two tooth types; premolar teeth (n=31) and molar teeth (n=3).
Tooth rotation
Tooth rotation was observed in 21.3% (10/47) of all examined teeth in the skulls. It was more common on the maxillae (n=8), in comparison to the mandibular counterparts (n=3). All rotations observed involved all the premolar teeth (P1=3, P2=4, P3=2, P4=2, respectively). Rotation was unilateral in all affected skulls, and the rotation angle varied but not more than 45°.
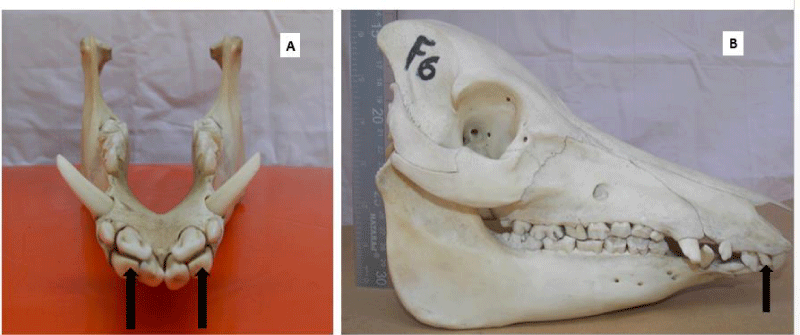
Persistent deciduous teeth
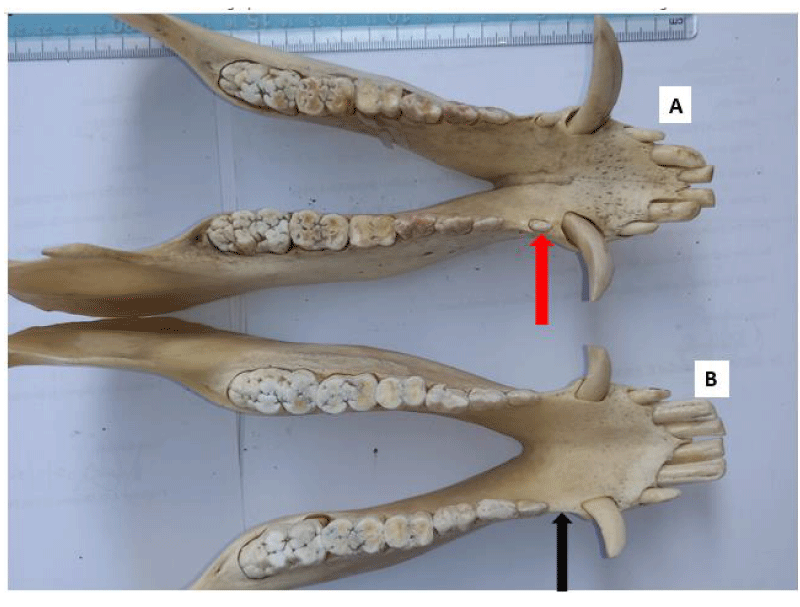
Persistent (retained) deciduous teeth were found in 21 of 23 adult skulls examined (91.3%: 9 males, 12 females). All the affected skulls had retained incisor teeth, which were bilateral in 81% of the affected skulls (17/21) and were located rostral to the permanent incisors (on the labial aspect). The deciduous second incisor was the most commonly retained tooth (n=25), while the first and third incisor teeth had 3 retained teeth each.
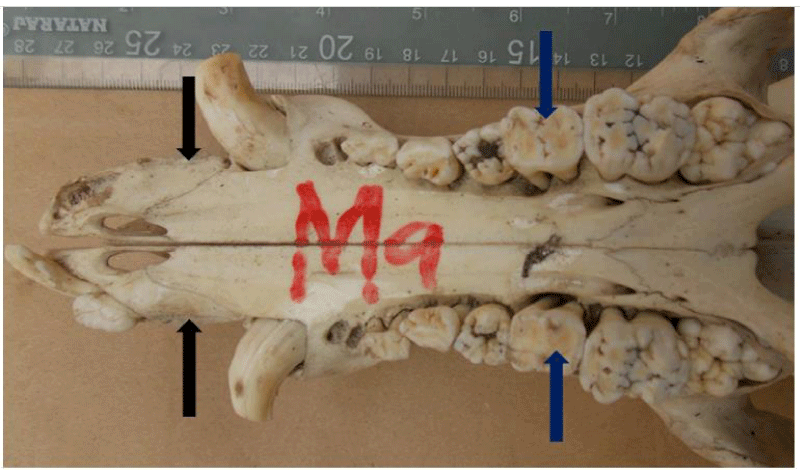
Missing teeth
A total of 66% of pig skulls (31/47) had at least a missing tooth, out of which 90.3% (28/31) had missing first premolar teeth, while only 9.7% (3/21) had missing third incisor teeth. The absence of mandibular first premolar tooth was more common in the females (100%, 17/17) while missing maxillary first premolar tooth was observed only in the males. Bilaterally missing first mandibular premolar teeth were observed in 75% (21/28) of examined skulls (6 males, 15 females), while 25% (7/28) had unilaterally missed first premolar teeth on the mandible and maxillae. Missing third incisor teeth were observed in 3 skulls; 66.7% (2/3) had no teeth alveoli (congenital absence) (1 bilateral, 1 unilateral) while 33.3% (1/3) was due to tooth loss.
Discussion
We have been able to identify and describe some dental pathologies in the teeth of the NLPs. Dental attrition was observed to be the most common dental pathology present in the skulls of the NLP. Dental attrition is caused by tooth-to-tooth contact or grinding of opposing teeth (bruxism), which leads to the formation of wear facets on the original enamel structure [27]. It can affect both deciduous and permanent teeth and the wear pattern of affected teeth is probably determined by the animal species and a combination of anatomical and functional characteristics of individual teeth [13,28]. The high prevalence of dental attrition observed in this study, affecting both deciduous and permanent teeth, was similar to what was reported in Swedish wild boars [13] and Finnish commercial sows [29]. In our study, the first molar tooth appeared to be the most severely affected, similar to what had been documented [13,30]. The first molars are the first permanent teeth to erupt, thus exposing them to attrition, over a longer period, when compared to other teeth. This may be responsible for the severity of attrition of the molar teeth found in this study. Conversely, the prevalence of attrition was higher in the incisor teeth, when compared to other teeth types [31] posited that this might be due to the thickness of the enamel on the incisors and the high rate of retention of the incisors in old people. Furthermore, since incisor teeth (especially the third) are also among the first deciduous and permanent teeth to erupt in pigs, they are often subjected to attrition over a long period [32]. Suggested that molar teeth wear was associated with aging, while incisor teeth wear could be due to some indoor factors such as bar biting or other abrasive actions. This is especially so because of the rostral positions of incisor teeth on the jaws. In affected animals, progressive tooth wear may lead to pulpitis and subsequent periodontitis which results in oral pain, dysfunction, and tooth loss [13,29].
Dental caries is a discolored and demineralized area on teeth surfaces that affects the enamel and dentin, and may ultimately affect the pulp and periodontal tissues. The demineralization is caused by organic acids produced by cariogenic bacteria in dental plaques, through the anaerobic metabolism of dietary carbohydrates [13,33]. If left unchecked, the process leads to the formation of the tooth cavity, which can lead to bacterial infection of the pulp and result in severe discomfort [13]. Although the development of dental caries is influenced by the diet, the susceptibility of the tooth, the bacterial profile, the quantity and quality of the saliva, and the availability of dietary carbohydrates for bacterial fermentation, may influence an animal’s susceptibility to the condition [13,33]. In this present study, a 34% prevalence was higher than the 2% reported in wild pigs [7], the 11% reported in Swedish wild boar [13], and the 5% reported in Swedish commercial pigs [25]. The cause of this high prevalence may be a genetic susceptibility of NLP to the disease and possibly, the effect of their diet. Although dental caries were not observed in piglet skulls in this study, the condition has been reported in piglets [16]. Observed that many piglets had discolored teeth at birth and developed dental caries afterward. This could relate to prenatal conditions, as dental caries development within weeks of birth shows that in-utero deposition of enamel was insufficient. Since enamel is laid over dentin for protection against environmental and nutritional insults during tooth formation, defects in this tissue are permanent and can greatly reduce the integrity and longevity of the tooth, which ultimately results in sub-optimal growth [16,34].
Dental calculus, a hard calcified plaque was observed in 74% of the skulls used for this study. This is higher than the 11% reported in commercial Swedish sows [25] and 40% in Swedish wild pigs [13]. These hard deposits were formed and found above the crowns of affected teeth, coronal to the gingiva margin (supragingival calculi). Calculus distribution differs across individuals, teeth, and surfaces [35]. In humans, the level and location of calculi formation are population-specific and are affected by oral hygiene habits, access to professional oral care, diet, age, frequency of cleaning, and the use of medication [36]. Dental calculus accumulation is reported to increase with the age of the animal [37]. This was evident in our study, where the teeth of older skulls had more deposits while very young skulls had none. Moderate and severe calculi were more prominent on the premolar teeth in older animals, similar to the report in Swedish wild pigs, where 93% of severely affected teeth were the premolars [13]. This suggests that calculus formation is progressive and infrequent use of teeth may encourage the formation of plaques and subsequent calculi.
Teeth are typically composed of several colors and a gradation of colors occurs in a particular tooth from its gingival margin to the incisal third of the tooth. The gingival margin is usually darker in appearance because of the closeness of the dentin below the enamel. In most cases, canine teeth are usually darker than central and lateral incisors [38,39]. These color variations may also be related to the thickness and translucency of enamel and dentin [40]. Teeth become darker as individual ages, probably due to the laying down of secondary and tertiary dentin, incorporation of extrinsic stains, and gradual enamel wear allowing a greater influence on the color of the underlying dentin [38,39]. In humans, dental staining is classified as being intrinsic, extrinsic, or “internalized discoloration”, depending on its depth of penetration within the tooth [34,38]. Intrinsic discoloration involves the deposition of chromogenic or hematological agents into the enamel or dentin matrix during development, illness, or trauma, while extrinsic stains develop when chromogenic agents, metal salts, or cationic antiseptics accumulate within the dental pellicle or the dental plaque. They become visible only after the eruption of the tooth. Internalized discoloration, however, refers to the changes in normal tooth color due to dental caries, tooth wears and gingival recession, cracks, and restorative materials [34,38-40]. The stains observed on the teeth of the skulls used for this study could be due to any of the aforementioned classifications.
Tooth fracture (cracked tooth) is a break in the enamel, dentin, or cementum of an affected tooth. It is a problem of increasing clinical significance, with many predisposing factors [41]. Fractures were observed in 47% of the examined skulls which was higher than the 8% reported in Swedish wild pigs [13], the 22% in domestic pigs [14], the 41% reported in Swedish commercial sows [25], and the 25% in Finnish commercial sows [29]. Although fractures have been categorized, based on type, location, and nature of the fracture, as either complete or incomplete [42], the fractures observed in this study were not classified. Unlike what was reported in Swedish wild boars and Swedish commercial sows respectively, where the incisor teeth were the most commonly fractured [13,25], our study revealed that the premolar teeth were the most affected (55%), while the incisor and molar teeth were the least affected (8%, respectively). In pigs, morphological and anatomical attributes of the teeth, diet, and behavioral routines (fighting, bar biting, and other aggressive traits) may incline certain teeth to fractures or cracks [13]. Dental caries have also been implicated as a predisposing factor for tooth fracture [43]. The 34% prevalence of dental caries observed in this study could also be responsible for the observed dental fractures. Our study showed that the frequency of fractures increased with age implying that permanent dentition could be more commonly affected compared to the deciduous dentition. The possibility of root fractures, secondary to dental fractures should also be considered as an outcome [13].
Tooth rotation, the displacement of a tooth from its normal alignment on the dental arcade, appears to be connected with overcrowding of teeth in the jaws due to genetic factors such as skull size and length, or to severe undernutrition, leading to malocclusion and misalignment of teeth on the mandible and the maxilla [7,10]. In our study, 21% of the examined skulls showed evidence of rotation, higher than the 10% and 13% reported in wild pigs, and the 13% reported in bush pigs (Potamochoerus spp) [7]. It was also higher than the 20% reported in wild boars and 10% in domestic pigs [8]. Our study also showed that rotation was more common on the maxillae and affected only the premolar teeth. This is similar to what has been reported [7,8]. Since there was no evidence of overcrowding of teeth on the jaws of our study samples, teeth rotation could be due to a shortening of the jaws or due to the relative sizes of the teeth on the jaws. This may ultimately result in abnormal attrition of affected teeth.
A persistent deciduous tooth is retained on the dental arcade beyond the time of its root resorption and eventual exfoliation. Deciduous tooth roots undergo gradual resorption as the eruption of the succeeding permanent tooth is initiated. It can also occur in the absence of the underlying permanent tooth [44,45]. Developmental absence of a permanent successor, impaction, abnormal position, and late eruption of successor’s teeth are some of the causes of teeth retention [45,46]. In our study, persistent deciduous incisor tooth was observed in 78% of the adult skulls examined, being higher in females. Bilateral symmetry of persistent deciduous tooth was present in 74% of the skulls and the second deciduous incisor tooth was the most commonly retained. This observation is similar to what was reported in commercial sows, with 15% of them having persistent deciduous incisor teeth [32]. A similar observation was made by [14], where 43% of the pigs examined had persistent deciduous teeth; the second maxillary incisor tooth was the most affected. Therefore, based on the previously published eruption dates in pigs [47], the deciduous teeth noted in our study would be “persistent”. The presence of retained deciduous teeth is regarded as a consequence of the failure of eruption, rather than its cause. In humans, persistent deciduous teeth have been reported to lead to some clinical problems including periodontitis, profound caries, aesthetic and phonetic problems, and ankylosis [46]. It may result in overcrowding, malocclusion and misalignment, irregular tooth wear, caries, and ultimately, periodontal disease, causing pain and discomfort to affected animals.
Hypodontia generally refers to the absence of one or more teeth, either in deciduous or permanent dentition, due to a developmental anomaly or loss by extraction. It is considered to be the most common craniofacial malformation in humans, occurring as part of a sporadic finding, as part of a genetic syndrome, or as part of a non-syndromic condition [48-50]. Hypodontia may be classified as “true” hypodontia (congenital absence) or “false” hypodontia (tooth loss by periodontitis or other causes) [13]. Possible aetiologies for “missing” teeth include congenital absence, (hypodontia or oligodontia), previous exfoliation resulting in a loss, and fracture below the gingival margin [51]. Missing teeth are more common in the permanent dentition than in the deciduous dentition and may not be evident in young, growing animals, since they only possess deciduous dentition [50]. It becomes noticeable in adults when they should have been replaced with a permanent adult dentition. However, when a deciduous tooth is congenitally missing, its permanent successor is likely to be missing too [12,49,50]. In this study, 66% of examined skulls missed at least a tooth. Of this, 90% had missing first premolar teeth and 10% had missing third incisor teeth. This 66% prevalence is similar to what was reported in Swedish wild pigs [13], where the absence of teeth was the most common dental anomaly, occurring in 69% of the examined skulls, especially the mandibular first premolar. In a study of 58 commercial sows, 59% of the sows missed at least one tooth, with 50% of the missing teeth being premolars [25]. Another study on the skulls of wild and domestic pigs reported that oligodontia was the most common anomaly, occurring in 23% of wild and 50% of domestic pigs. Loss of the first lower premolar tooth occurred in 22 of the affected skulls [7,8]. however, reported a prevalence of 42% for missing maxillary third incisor teeth in one of the study samples.
Although no radiographic examinations were conducted on our study skulls, several reports of the incidence of missing first mandibular premolar teeth in wild and domestic pigs corroborate our report. Its position is highly variable and its pattern of eruption and relative isolation in the dental arcades may contribute to its frequency of being missing [8]. A widespread absence of the first mandibular premolar tooth could imply that it serves no masticatory function in adult life while the missing maxillary first premolar and third incisor teeth could be attributed to tooth loss secondary to periodontitis.
In conclusion, it has been established that NLPs are susceptible to and have dental pathologies. The current study described the presence and distribution of some dental pathologies. The study revealed that these dental pathologies affected all age groups, with the older skulls having the highest prevalence. These abnormalities were also observed to be more common in the female skulls. The data obtained from this study will be particularly important to pig farmers as a reference, as early detection of dental abnormalities can help promote productivity and reduces economic losses in pig husbandry. It is therefore important to know the types of dental disorders that affect pigs, the susceptible age groups, the frequency of occurrence, and the predisposing factors. Data obtained will also be useful to researchers, especially those using pigs in Nigeria as a model for translation research and comparative dental studies.
The authors are grateful to the management of Teaching and Research Farm, University of Ibadan, Dr. Ayodeji Lijoka of the department of Veterinary Anatomy, and Mr. Jacob Olayemi, for their support.
- Chen D, Blom H, Sanchez S, Tafforeau P, Märss T, Ahlberg PE. The developmental relationship between teeth and dermal odontodes in the most primitive bony fish Lophosteus. Elife. 2020 Dec 15;9:e60985. doi: 10.7554/eLife.60985. PMID: 33317696; PMCID: PMC7738188.
- Sharpe PT. Fish scale development: Hair today, teeth and scales yesterday? Curr Biol. 2001 Sep 18;11(18):R751-2. doi: 10.1016/s0960-9822(01)00438-9. PMID: 11566120.
- Papagerakis P, Mitsiadis T. Development and Structure of Teeth and Periodontal Tissues. In: Rosen CJM, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Eighth Edi. John Wiley & Sons, Inc; 904-913. 2013;
- Bailleul-Forestier I, Molla M, Verloes A, Berdal A. The genetic basis of inherited anomalies of the teeth. Part 1: clinical and molecular aspects of non-syndromic dental disorders. Eur J Med Genet. 2008 Jul-Aug;51(4):273-91. doi: 10.1016/j.ejmg.2008.02.009. Epub 2008 Mar 26. PMID: 18499550.
- Pineda-munoz S, Lazagabaster IA, Alroy J, Evans AR. Inferring diet from dental morphology in terrestrial mammals. Methods Ecol Evol. 2017; 8:481-491.
- Ungar PS. Mammalian dental function and wear: A review. Biosurface and Biotribology. 2005; 1(1):25-41.
- Horwitz L, Davidovitz G. Dental pathology of wild pigs (Sus scrofa) from Israel. Isr J Zool. 1992; 38(2):111-123.
- Feldhamer GAA, McCann BE. Dental anomalies in wild and domestic Sus scrofa in Illinois. Acta Theriol (Warsz). 2004; 49(1):139-143.
- Zinoviev AV. A supernumerary permanent mandibular premolar of wild boar (Sus scrofa L.) from the early medieval Novgorod, Russia. Int J Osteoarchaeol. 2010; 20(5):586-590.
- Binois A, Bridault A, Pion G, Ducrocq T. Dental development pathology in wild artiodactyls: Two prehistoric case studies from France. Int J Paleopathol. 2014 Mar;4:53-58. doi: 10.1016/j.ijpp.2013.11.002. Epub 2013 Dec 21. PMID: 29539502.
- Penzhorn BL. Dental abnormalities in free-ranging Cape mountain zebras (Equus zebra zebra). J Wildl Dis. 1984 Apr;20(2):161-6. doi: 10.7589/0090-3558-20.2.161. PMID: 6737614.
- Pavlica Z, Erjavec V, Petelin M. Teeth abnormalities in the dog. Acta Vet Brno. 2001; 70(1):65-72.
- Malmsten A, Dalin AM, Pettersson A. Caries, Periodontal Disease, Supernumerary Teeth and Other Dental Disorders in Swedish Wild Boar (Sus scrofa). J Comp Pathol. 2015 Jul;153(1):50-7. doi: 10.1016/j.jcpa.2015.04.003. Epub 2015 May 12. PMID: 25979683.
- Smith MA, Rao S, Rawlinson JE. Dental Pathology of the Domestic Pig (Sus Scrofa Domesticus). J Vet Dent. 2020 Dec;37(4):192-200. doi: 10.1177/0898756421989097. Epub 2021 Feb 19. PMID: 33601925.
- Štembírek J, Kyllar M, Putnová I, Stehlík L, Buchtová M. The pig as an experimental model for clinical craniofacial research. Lab Anim. 2012 Oct;46(4):269-79. doi: 10.1258/la.2012.012062. Epub 2012 Sep 11. PMID: 22969144.
- Tucker AL, Widowski TM. Normal profiles for deciduous dental eruption in domestic piglets: effect of sow, litter, and piglet characteristics. J Anim Sci. 2009 Jul;87(7):2274-81. doi: 10.2527/jas.2008-1498. Epub 2009 Mar 27. PMID: 19329477.
- Larsen MO, Rolin B. Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J. 2004;45(3):303-13. doi: 10.1093/ilar.45.3.303. PMID: 15229377.
- Wang S, Liu Y, Fang D, Shi S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007 Nov;13(6):530-7. doi: 10.1111/j.1601-0825.2006.01337.x. PMID: 17944668.
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006 Dec 20;1(1):e79. doi: 10.1371/journal.pone.0000079. PMID: 17183711; PMCID: PMC1762318.
- Nkenke E, Lehner B, Fenner M, Roman FS, Thams U, Neukam FW, Radespiel-Tröger M. Immediate versus delayed loading of dental implants in the maxillae of minipigs: follow-up of implant stability and implant failures. Int J Oral Maxillofac Implants. 2005 Jan-Feb;20(1):39-47. PMID: 15747672.
- Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010 Oct;28(10):1829-38. doi: 10.1002/stem.512. PMID: 20979138; PMCID: PMC2996858.
- Wang F, Xiao J, Cong W, Li A, Song T, Wei F, Xu J, Zhang C, Fan Z, Wang S. Morphology and chronology of diphyodont dentition in miniature pigs, Sus Scrofa. Oral Dis. 2014 May;20(4):367-79. doi: 10.1111/odi.12126. Epub 2013 May 16. PMID: 23679230.
- Adeola AC, Oseni SO, Omitogun OG. Morphological characterization of indigenous and crossbred pigs in rural and peri-urban areas of southwestern Nigeria. Open J Anim Sci. 2013; 3(3):230-235.
- AU-IBAR. Local African Pig. African Union Inter-african Bur Anim Resour. Published online 2015.
- Malmsten A, Lundeheim N, Dalin AM. Dental disorders in sows from Swedish commercial herds. Acta Vet Scand. 2020 Jun 4;62(1):27. doi: 10.1186/s13028-020-00521-7. PMID: 32498715; PMCID: PMC7273662.
- Olopade JO, Okandeji ME. A study of some rostrofacial indices related to regional anaesthesia of the porcine: implications as an animal model for dental research. Niger J Physiol Sci. 2010 Nov 28;25(2):159-64. PMID: 22314955.
- Sperber GH. Dental Wear: Attrition, Erosion, and Abrasion-A Palaeo-Odontological Approach. Dent J (Basel). 2017 Jun 17;5(2):19. doi: 10.3390/dj5020019. PMID: 29563425; PMCID: PMC5806976.
- kene ROC, Uwagie-Ero EA. Dental Abnormalities of Nomadic Cattle of Nigeria. Trop Vet. 2001; 19(3):191-199.
- Ala-Kurikka E, Munsterhjelm C, Bergman P, Laine T, Pekkarinen H, Peltoniemi O, Valros A, Heinonen M. Pathological findings in spontaneously dead and euthanized sows - a descriptive study. Porcine Health Manag. 2019 Nov 20;5:25. doi: 10.1186/s40813-019-0132-y. PMID: 31832226; PMCID: PMC6864960.
- Samuel JL, Woodall PF. Periodontal disease in feral pigs (Sus scrofa) from Queensland, Australia. J Wildl Dis. 1988 Apr;24(2):201-6. doi: 10.7589/0090-3558-24.2.201. PMID: 3373625.
- Sun K, Wang W, Wang X, Shi X, Si Y, Zheng S. Tooth wear: a cross-sectional investigation of the prevalence and risk factors in Beijing, China. BDJ Open. 2017 Jan 27;3:16012. doi: 10.1038/bdjopen.2016.12. PMID: 29607073; PMCID: PMC5842827.
- Johnson EW, Curtis SE, Ellis M. Dental Disease in Sows - Early Findings. Swine Conf. 2003.
- Moynihan P, Petersen PE. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004 Feb;7(1A):201-26. doi: 10.1079/phn2003589. PMID: 14972061.
- Tucker AL, Farzan A, Cassar G, Friendship RM. Effect of in-water iodine supplementation on weight gain, diarrhea and oral and dental health of nursery pigs. Can J Vet Res. 2011 Oct;75(4):292-7. PMID: 22468027; PMCID: PMC3187636.
- Aghanashini S, Puvvalla B, Mundinamane DB, Apoorva S, Bhat D, et al. Comprehensive Review on Dental Calculus. J Heal Sci Res. 2016; 7(2):42-50.
- White DJ. Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur J Oral Sci. 1997 Oct;105(5 Pt 2):508-22. doi: 10.1111/j.1600-0722.1997.tb00238.x. PMID: 9395117.
- Kyllar M, Witter K. Prevalence of dental disorders in pet dogs. Vet Med (Praha). 2005; 50(11):496-505.
- Mortazavi H, Baharvand M, Khodadoustan A. Colors in tooth discoloration: A new classification and literature review. Int J Clin Dent. 2014; 7(1):17-28. Link:
- Watts A, Addy M. Tooth discolouration and staining: a review of the literature. Br Dent J. 2001 Mar 24;190(6):309-16. doi: 10.1038/sj.bdj.4800959. PMID: 11325156.
- Sulieman M. An overview of tooth discoloration: extrinsic, intrinsic and internalized stains. Dent Update. 2005 Oct;32(8):463-4, 466-8, 471. doi: 10.12968/denu.2005.32.8.463. PMID: 16262034.
- Lynch CD, McConnell RJ. The cracked tooth syndrome. J Can Dent Assoc. 2002 Sep;68(8):470-5. PMID: 12323102.
- Ellis SG. Incomplete tooth fracture--proposal for a new definition. Br Dent J. 2001 Apr 28;190(8):424-8. doi: 10.1038/sj.bdj.4800992. PMID: 11352390.
- Soares TR, Fidalgo TK, Quirino AS, Ferreira DM, Chianca TK, Risso PA, Maia LC. Is caries a risk factor for dental trauma? A systematic review and meta-analysis. Dent Traumatol. 2017 Feb;33(1):4-12. doi: 10.1111/edt.12295. Epub 2016 Jul 20. PMID: 27439566.
- Kjaer I, Nielsen MH, Skovgaard LT. Can persistence of primary molars be predicted in subjects with multiple tooth agenesis? Eur J Orthod. 2008 Jun;30(3):249-53. doi: 10.1093/ejo/cjm123. PMID: 18540013.
- Aktan AM, Kara I, Sener I, Bereket C, Celik S, Kirtay M, Ciftçi ME, Arici N. An evaluation of factors associated with persistent primary teeth. Eur J Orthod. 2012 Apr;34(2):208-12. doi: 10.1093/ejo/cjq189. Epub 2011 Jan 12. PMID: 21228121.
- Deepa D, Rana N, Arun Kumar K. Nonsyndromic bilateral multiple retained primary incisors in mandibular arch: Rare case report. J Oral Res Rev. 2006; 8(2):79.
- Matschke GH. Aging European wild hogs by dentition. J Wildl Manage. 1967; 31(1):109-113.
- Wu CC-L, Wong RW-K, Hägg U. A review of hypodontia: the possible etiologies and orthodontic, surgical and restorative treatment options-conventional and futuristic. Hong Kong Dent J. 2007; 4(2):113-121.
- Klein OD, Oberoi S, Huysseune A, Hovorakova M, Peterka M, Peterkova R. Developmental disorders of the dentition: an update. Am J Med Genet C Semin Med Genet. 2013 Nov;163C(4):318-32. doi: 10.1002/ajmg.c.31382. Epub 2013 Oct 4. PMID: 24124058; PMCID: PMC3844689.
- Al-Ani AH, Antoun JS, Thomson WM, Merriman TR, Farella M. Hypodontia: An Update on Its Etiology, Classification, and Clinical Management. Biomed Res Int. 2017.
- Arte S, Pirinen S. Hypodontia. (Bloch-Zupan A, ed.). Orphanet; 2004.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
