Ann Cytol Pathol
Immunohistochemical Analysis and Pathological Assessment of B-Cbl Proto-Oncogene in Gastric Carcinoma Cells
Rachida Salah1, Noria Harir1,2*, Feriel Sellam1, Malika Aidouni3, Miloud Medjamia3, Nesrine Mrabent1, Soumia Zeggai1 and Mustapha Diaf1
2Laboratory of Molecular Microbiology, Proteomics and Health; Sidi bel Abbes, Algeria
3Department of Pathology; Oran Military Hospital (HMRUO), Algeria
Cite this as
Salah R, Harir N, Sellam F, Aidouni M, Medjamia M, et al. (2016) Immunohistochemical Analysis and Pathological Assessment of B-Cbl Proto-Oncogene in Gastric Carcinoma Cells. Ann Cytol Pathol 1(1): 054-057. DOI: 10.17352/acp.000008Objective: In order to study the status and the expression of Cbl-b onco-protein in gastric carcinoma we underwent an immunohistochemical protocol by which we analyzed the immuno-distribution as well as the level of Cbl-b expression on gastric cancerous tissues.
Material and method: Ninety-six (96) paraffin blocks of cancerous gastric tissues sections were collected from the Department of pathology, of Oran Military Hospital (Western Algeria) for the period of 2006-2015. All the cases were identified histologically as malignant gastric cells. An immunohistochemical protocol to analyze the expression of Cbl-b in gastric cancerous tissues.
Results: Our immunohistochemical analysis revealed that Cbl-b protein was expressed in gastric cancerous cells with different immune-scoring and different immunostaining intensity. The most predominant observed score was score 0 in 37 cases (38.5%) followed by score 2 in 13 cases (13.5%); then score 4 in 11 cases (11.5%). The least predominant scores were score 6 in 7 cases (7.3%), than score 1, 5 and 7 with the same number of cases 6 (6.3%). Concerning immunostaining levels; we reported an intense staining in 14 cases (14.6%); a moderate staining in 16 cases (16.7%); a poor staining in 14 cases (14.6%) and no staining in 52 cases (54.2%).
Conclusion: Cbl-b oncoprotein is immunohistochemically expressed in human gastric carcinoma. More surveys are required to determine the role of Cbl-b in non-hematopoeitic cell systems.
Introduction
Gastric cancer is becoming a major health problem worldwide because of the lack of effective biomarkers for metastasis prediction [1].
The mammalian Cbl protein down regulation was implicated in gastric cancer development [2]. Cbl family consists of the three homologues c-Cbl, Cbl-b, and Cbl-3, all of which associate with a wide variety of signaling proteins [3].
Through these many associations, Cbl is able to regulate diverse signaling networks; one of the most extensively studied roles of Cbl is its function as a negative regulator of receptor tyrosine kinase (RTK) signaling [4-6].
Cbl-b is a second member of the E3 ubiquitin ligase Cbl family, some studies have revealed that Cbl-b regulates gastric cancer cell proliferation, drug sensitivity and also migration [1,7,8]. In addition, Cbl-b can degrade as well IGF-I signaling intermediate IRS-1 and decrease protein synthesis in unloading-induced muscle atrophy [9]. Some surveys showed that the p85 regulatory subunit of PI3 kinase (PI3K) serves a substrate for Cbl-b during activation of T cells and Cbl-b functions as a negative regulator of p85 in a ubiquitin-dependent but proteolysis-independent manner [10,11]. Cbl-b-deficient inhibited Akt and ERK phosphorylation and deregulation of T cell proliferation [12,14].
In order to study the status and expression of Cbl-b oncoprotein in gastric carcinoma we underwent an immunohistochemical protocol by which we analyzed the immuno-distribution as well as the level of Cbl-b expression on gastric cancerous tissues.
Material and Methods
Ninety-six (96) paraffin blocks of cancerous gastric tissues sections were collected from the Department of pathology of Oran Military Hospital (Western Algeria) for the period of 2006-2015. All the cases were identified histologically as malignant gastric cells. These specimens were paraffin embedded in the same center. The cases were stained with hemotoxyline and eosin (H&E) for routine histological examination. An absolute confidentiality of the patients’ vital information was maintained for ethical purposes and an ethical approval was obtained from the institution in which the study was carried out.
Immunohistochemical protocol
We used the following Abcam protocol of immunohistochemistry (IHC): formalin-fixed paraffin-embedded tissue sections were deparaffinized and rehydrated then enough drops of Hydrogen Peroxide Block were added to cover the sections, they were incubated for 10 minutes then washed twice in buffer Phosphate Buffered Saline (PBS) (10X PBS) (0.1M PBS, pH 7.4).
A pretreatment was performed then the sections were washed 3 times in buffer.
Protein Block was applied and the sections were incubated for 10 minutes at room temperature to block nonspecific background staining then washed once in buffer. Anti-Cbl-b antibody was applied (1:100 dilution mouse monocloal; BD Biosciences, CA) then incubated overnight. Tissues were washed 3 times in buffer then complement was applied and incubated for 10 minutes at room temperature. Sections were washed twice in buffer then HRP conjugate was applied and incubated for 15 minutes at room temperature; they were rinsed 4 times in buffer, then 30 μl (1 drop) DAB Chromogen were added to 1.5 ml (50 drops) of DAB Substrate and were applied to tissue and were incubated for 1-10 minutes then rinsed 4 times in buffer. Counterstain was applied then the tissues were dehydrated.
Morphometric evaluation
Concerning IHC control; we followed Dong et al. [4], scale for the assessment: positive staining of cCbl, was indicated as yellowish brown granules in the cytomembrane, the cytoplasm, or both. A pathologist observed sections through microscopic examination (x400). From each section, five visual fields were randomly selected and the score for each visual field depended on its percent of positive cells and their staining intensity. For the percent of positive cells, less or equal to 5%, 6%25%, 26% 50%, 51%75% and more than 76% were recorded as 0, 1, 2, 3, and 4 points, respectively. For staining intensity, non-stained, light yellow, yellowish brown, and brown were recorded as 0, 1, 2, and 3 points, respectively. The arithmetic product of these two scores (percent score and intensity score) was regarded as the score for that visual field, and the average score of those five visual fields were regarded as the final score for that section. Finally, 01 point was recorded as (-), which indicated negative staining; 24 points were recorded as (+), which indicated weak positive staining; 57 points were recorded as (++) and less or equal to 8 points as (+++), which indicated strong positive staining. Final scores were assigned by a pathologist.
Statistical analysis
Statistical analyses were done using SPSS 20.0 (Statistical Package for the Social Sciences, IBM Corporation; Chicago, IL. August 2011).
Results
96 patients with gastric cancer were included in the current study. Median age of the patients with gastric cancer was 60 years (range, 27-87). There were 70 (72.9 %) males and 26 (27.1%) females; male to female ratio was about 2.7 (Table 1).
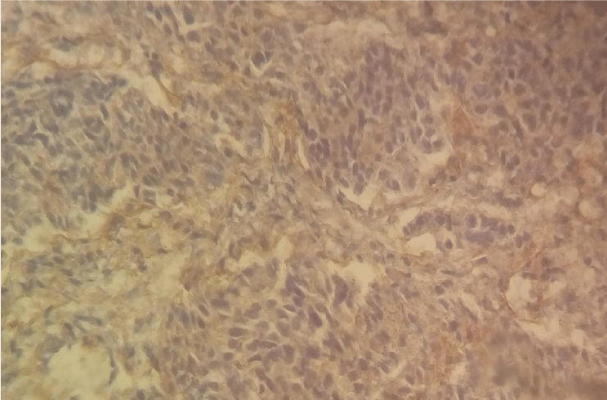
As shown in Table 1 the most common histological type was moderately differentiated adenocarcinoma 33 cases (34.4%) (Figure 1), well differentiated adenocarcinoma 24 cases (25%) (Figure 2), signet-ring cell carcinoma 20 (20.8%); poorly differentiated adenocarcinoma 15 cases (15.6%) (Figure 3), and colloids mucosal carcinoma 4 (4.2%).
Our immunohistochemical analysis revealed that Cbl-b protein was expressed in gastric cancerous cells but with different of immune-scoring and different immunostaining intensity.
The most predominant observed score was score 0 in 37 cases (38.5%) followed by score 2 in 13 cases (13.5%) (Figure 3), then score 4 in 11 cases (11.5%). The least predominant scores were score 6 in 7 cases (7.3%) (Figure 2), than score 1, 5 (Figure 1) and 7 with the same number of cases 6 (6.3%) (Table 2).
Concerning immunostaining levels, we reported an intense staining in 14 cases (14.6%); a moderate staining in 16 cases (16.7%); a poor staining in 14 cases (14.6%) and no staining in 52 cases (54.2%) (Table 3).
Discussion
In the present study, we examined the immuno-expression as well as the immunodistribution of the proto-oncogene b-Cbl in human gastric carcinoma using an immunoistochemical protocol. The expression of b-Cbl protein was detected in 45.8 % of gastric carcinomas, and overexpressed in 19 cases (score 5, 6, 7) with a rate of 19.7% which is a prominent rate.
As we stated before; Cbl-b can be an important regulator of several signaling pathways. This oncoprotein might act also as a negative regulator of growth factor receptor signaling and receptor tyrosine kinase (RTK) signaling, which make the ubiquitin ligase Cbl-b play an important role in the suppression of gastric cancer cell proliferation [4-6].
Two highly conserved amino-terminal domains contribute strongly to this regulatory function. First, Cbl’s tyrosine kinase binding (TKB) domain recognizes phosphotyrosine residues and allows Cbl to interact directly with activated RTKs on the plasma membrane [14-16].
The loss of Cbl-b in T cells was demonstrated to trigger antigen-induced receptor clustering and lipid raft aggregation [17]. Some previous surveys showed that Cbl-b sequestered signalling molecules from lipid rafts, which resulted in ineffective lipid raft aggregation in mast cells [18,19].
Furthermore, down-regulation of Cbl-b by oxaliplatin was shown to facilitate death receptor aggregation in lipid rafts in gastric cancer cells [20]. Therefore, since lipid rafts are essential participants in the regulation of receptor-mediated signal transduction and cross-talk in membrane microdomains [21,22].
Some authors stated that knock-down of Cbl-b enhances epidermal growth factor-induced disruption of human epithelial cell adherens junctions (AJs) and cell motility [22]. The inducible up-regulation of c-Cbl and Cbl-b affects cell adhesion through regulation of the adhesion-related kinases Pyk2 and Paxillin in HL-60 cell differentiation [23]. Moreover, Cbl-b can also degrade the IGF-I signaling intermediate IRS-1 and reduce protein synthesis in unloading-induced muscle atrophy [24-26].
Feng et al. [13], suggested that Cbl-b functions together with EGFR/ERK/Akt pathway regulating the balance between chemosensitivity and chemoresistance to 5-Fluorouracil (5-FU), have shown that the chemotherapeutic agent, oxaliplatin, can sensitize gastric cancer cells to TRAIL by regulating components of the apototic/survival machinery such as caspase-3, caspase-8, Bax, and Bcl-2 protein expression [13,23]. These studies support an involvement of Cbl-b and its potential regulation of EGFR signaling in determining chemosensitivity of gastric cancer cells. The knockdown studies of Cbl-b and biochemical analyses of EGFR signaling in the present study directly support this idea [13].
Conclusion
The present study has shown that Cbl-b oncoprotein is immunohistochemically expressed in human gastric carcinoma. More surveys are required to determine the role of Cbl-b in non-hematopoeitic cell systems using several molecular techniques such as RT-PCR and western blotting.
- Li H, Xu L, Li C, Zhao L, Ma Y, et al. (2014) Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Molecular Cancer 13: 136 .
- Schmidt MH, Dikic I (2005) The Cbl interactome and its functions. Nat Rev Mol Cell Biol 6: 907-918 .
- Thien CB, Langdon WY (2001) Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol 2: 294–307 .
- Miyake S, Lupher ML Jr, Druker B, Band H (1988) The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc Natl Acad Sci USA 95: 7927–7932 .
- Ota Y, Samelson LE (1997) The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science 276: 418–420 .
- Waterman H, Levkowitz G, Alroy I, Yarden Y (1999) The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J Biol Chem 274: 22151–22154 .
- Yingchun L, Xiujuan Q, Jinglei Q, Ye Z, Jing L, et al. (2011) E3 ubiquitin ligase Cbl-b potentiates the apoptotic action of arsenic trioxide by inhibiting the PI3K/Akt pathway. Braz J Med Biol Res 44: 105–111 .
- Xu L, Zhang Y, Liu J, Qu J, Hu X, et al. (2012) TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonizes TRAIL-induced apoptosis in gastric cancer cells. Eur J Cancer 48: 3288–3299 .
- Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, et al. (2009) Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol 29: 4798–4811 .
- Fang D, Wang HY, Fang N, Altman Y, Elly C, et al. (2001) Cbl-b, a RING-type E3 ubiquitin ligase,targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem276: 4872–4878 .
- Fang D, Liu YC (2001) Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat Immunol 2: 870–875 .
- Zhang R, Zhang N, Mueller DL (2008) Casitas B-lineage lymphoma b inhibits antigen recognition and slows cell cycle progression at late times during CD4+ T cell clonal expansion. J Immunol 181: 5331-5339 .
- Feng D, Ma Y, Liu J, Xu L, Zhang Y, et al. (2013) Cbl-b Enhances Sensitivity to 5-Fluorouracil via EGFR- and Mitochondria-Mediated Pathways in Gastric Cancer Cells. Int J Mol Sci 14: 24399-24411 .
- Galisteo ML, Dikic I, Batzer AG, Langdon WY, Schlessinger J (1995) Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem 270: 20242-20245 .
- Lupher ML Jr, Reedquist KA, Miyake S, Langdon WY, Band H (1996) A novel phosphotyrosine-binding domain in the N-terminal transforming region of Cbl interacts directly and selectively with ZAP-70 in T cells. J Biol Chem 271: 24063-24068 .
- Wang Y, Yeung YG, Langdon WY, Stanley ER (1996) c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J Biol Chem 271: 17-20 .
- Krawczyk C, Bachmaier K, Sasaki T, Jones RG, Snapper SB, et al. (2000) Cbl-b is a negative regulator of receptor clustering and raft aggregation in T cells. Immunity 13: 463-473 .
- Qu X, Miah SM, Hatani T, Okazaki M, Hori-Tamura N, et al. (2005) Selective inhibition of FcRI-mediated mast cell activation by a truncated variant of Cbl-b related to the rat model of type 1 diabetes mellitus. J Biochem 137: 711-720 .
- Qu X, Sada K, Kyo S, Maeno K, Shahjahan Miah SM, et al. (2004) Negative regulation of Fcepsilon RImediated mast cell activation by a ubiquitin-protein ligase Cbl-b. Blood 103: 1779-1786 .
- Xu L, Qu X, Zhang Y, Hu X, Yang X, et al. (2009) Oxaliplatin enhances TRAIL-induced apoptosis in gastric cancer cells by CBL-regulated death receptor redistribution in lipid rafts. FEBS Lett 583: 943-948 .
- Hur EM, Park YS, Lee BD, Jang IH, Kim HS, et al. (2004) Sensitization of epidermal growth factor-induced signaling by bradykinin is mediated by c- Src. Implications for a role of lipid microdomains. J Biol Chem 279: 5852-5860 .
- Xu L, Zhang Y, Liu J, Qu J, Hu X, et al. (2012) TRAIL-activated EGFR by Cbl-b-regulated EGFR redistribution in lipid rafts antagonises TRAIL-induced apoptosis in gastric cancer cells. European Journal of Cancer 48: 3288-3299 .
- Duan L, Raja SM, Chen G, Virmani S, Williams SH, et al. (2011) Negative regulation of EGFR-Vav2 signaling axis by Cbl ubiquitin ligas controls EGF receptor-mediated epithelial cell adherens junction dynamics and cell migration. J Biol Chem 286: 620-633 .
- Qu X, Liu Y, Ma Y, Zhang Y, Li Y, et al. (2008) Up-regulation of the Cbl family of ubiquitin ligases is involved in ATRA and bufalin-induced cell adhesion but not cell differentiation. Biochem Biophys Res Commun 367:183-189 .
- Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, et al. (2009) Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol 29: 4798-4811 .
- Xu L, Qu X, Zhang Y, Hu X, Yang X, et al. (2009) Oxaliplatin enhances TRAIL-induced apoptosis in gastric cancer cells by CBL-regulated death receptor redistribution in lipid rafts. FEBS Lett 583: 943-948 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
