Ann Cytol Pathol
Sex-determining region y (SRY)-Box2 (SOX2) & L1-cell adhesion molecule (L1CAM) expressions in endometrioid carcinoma of the uterus; An Immunohistochemical Study
Amir M Salem1* and Entsar R Mahdy2
2Lecturer of Gynecology and Obstetrics, Faculty of Medicine, Zagazig University, Egypt
Cite this as
Salem AM, Mahdy ER (2018) Sex-determining region y (SRY)-Box2 (SOX2) & L1-cell adhesion molecule (L1CAM) expressions in endometrioid carcinoma of the uterus; An Immunohistochemical Study. Ann Cytol Pathol 2(1): 001-008. DOI: 10.17352/acp.000010Background: activation of cancer stem cells and disturbances in cell adhesion pathways are recently incriminated in endometrioid carcinoma progression, invasion and metastases which consequently leads to dismal patients outcome. Sex-determining region y (SRY)-Box2 (SOX2) is a member of SOX family and it has many roles in malignant stem cells control. L1-cell adhesion molecule (L1CAM) is a membrane glycoprotein which is a member of immunoglobulin family.
The aim of the work: is to evaluate prognostic, clinical and pathological values of Sox2& L1CAM expression in tissues of endometrioid carcinoma of the uterus.
Patients and methods: Sox2& L1CAM tissue protein expression was assessed by immunohistochemistry in sections from sixty paraffin blocks which are retrieved from 60 patients with endometrioid carcinoma of the uterus then correlations between their levels of expression, clinical, pathological data, progression and recurrence of the tumor and patients outcome were analyzed.
Results: SOX-2 expression in endometrioid carcinoma was associated with old age of the patient (p=0.003), larger tumor size(p=0.004), higher grade, advanced stage, presence of L.N and distant metastases (p<0.001), presence of myometrial invasion, cervical stromal invasion (p=0.006), lymphovascular (p=0.02) & parametrial (p=0.007), serosal (p=0.03), and adnexal invasions (p=0.008), shorter 5-year overall survival rate (p=0.003), and shorter 5-year disease free survival rate (p=0.005).
L1CAM expression in endometrioid carcinoma was associated with larger tumor size (p=0.017), higher grade (p=0.0 43),, advanced stage(p=0.0 31), presence of L.N (p=0.0 33), and distant metastases(p=0.0 49), presence of myometrial invasion (p=0.0 27), cervical stromal invasion (p=0.026), lymphovascular (p=0.02) & parametrial (p=0.012), serosal (p=0.042), and adnexal invasions (p=0.034), shorter 5-year overall survival rate (p=0.002), and shorter 5-year disease free survival rate (p=0.006). We found a positive relationship between SOX-2 and L1CAM r (Correlation Coefficient) = +0.735 (P<0.001).
Conclusion: Sox-2& L1CAM expression correlates with poor clinicopathological parameters of endometrioid carcinoma
Introduction
Malignant epithelial tumor which originated from the endometrium is named as endometrial carcinoma (EC) and it forms about 20-30% of cancer of the female gynecological malignancies. Due to high incidence of hypertension, obesity, diabetes and increased life span; EC incidence and fatality has been markedly increased [1]. Moreover, endometrial carcinoma is still ranking the commonest gynecologic malignancy in developed countries and the second commonest in the developing countries, while, cancer cervix is still the first [2]. The majority of endometrial carcinomas are classified as type I endometrioid endometrial carcinoma and type II non-endometrioid endometrial carcinoma [3]. Endometrioid carcinoma is the commonest subtype of endometrial carcinoma [4]. Recently the role of stem cells, especially cancer stem cells has gained attention to be responsible for progression, invasion and spread of several malignancies [5,6]. Genetic mutations which could derive cancer occurrence happen over years, and only cancer stem cells which were found to have long life span is incriminated to accumulate the genetic mutations that are sufficient to initiate cancer and leads to malignant progression. So, cancer stem cells are responsible for invasion, carcinogenesis and spread of cancer cells in different organs [5]. Data about role of cancer stem cells in malignant progression have directed researchers to detect novel targeted therapies against these cells, which lead to improvement therapeutic response and patients’ outcome, there are so many biomarkers that are responsible for identification of cancer stem cells, studying and targeted them is the recent hope for improving cancer management [7]. Sex-determining-region-y (SRY)-Box2 (SOX-2) is a member of SOX family of transcription factors which plays an essential role in controlling cancer stem cell properties [8,9]. The disturbances in adhesion of malignant epithelial cells are responsible for invasion and spread of those cells so studying the detailed role of adhesion molecules in cancer helps to detect its pathogenesis and detection of new therapeutic targets that decreased invasion and metastases. L1-cell adhesion molecule (L1CAM) is considered a membrane glycoprotein which is an immunoglobulin superfamily member and it is involved in neurogenesis processes [10]. In addition, to its physiological role it plays an essential role in progression of many tumors [11].
The aim of the work; is to evaluate prognostic, clinical and pathological values of Sox2& L1CAM expression in tissues of endometroid carcinoma of the uterus.
Patients and Methods
We have performed the current study on a cohort of sixty patients of endometrioid carcinoma that were surgically managed in Gynecology and Obstetrics Department Faculty of Medicine Zagazig University and the specimen sent to Pathology Department Faculty of Medicine Zagazig University where we have prepared 60 paraffin block of endometroid carcinoma in the period from May 2013 to May 2018. All data were collected from patients file and completed by examinations of all slides. To stage and grade all cases we have used international-federation of gynecology and obstetrics’ (FIGO) staging system [12]. We have evaluated SOX-2 and L1CAM expressions using immunohistochemistry in sections prepared from all the 60 paraffin blocks correlate their expression with clinic-pathological parameters and follow-up data. We have followed up patients for 5 years, follow up and survival data were collected from Oncology Departments, Faculty of medicine, Zagazig University. Follow up dead line was May 2018. Adjuvant radio-therapy with or without platinum-based chemotherapy was given according to surgical staging.
Immunohistochemical staining
We used Streptavidine-biotin technique for immunohistochemical staining [13]. Sections were incubated overnight with primary anti-mouse monoclonal antibodies; anti-SOX2 antibody (ab171380) & Anti-L1CAM antibody [2C2] (ab24345) (abcam, UK). We used section from human lung squamous carcinoma tissue and nervous system tissue as an external positive control for SOX-2& L1CAM respectively.
Evaluation of immunohistochemical expression of SOX-2& L1CAM:
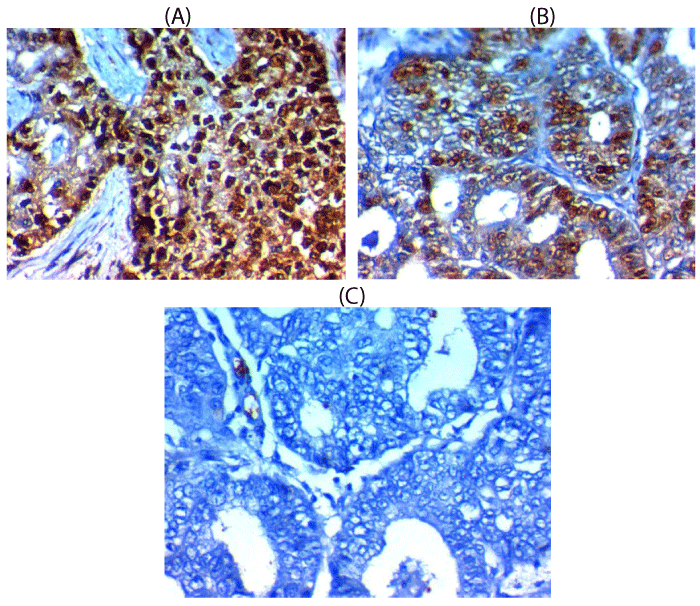
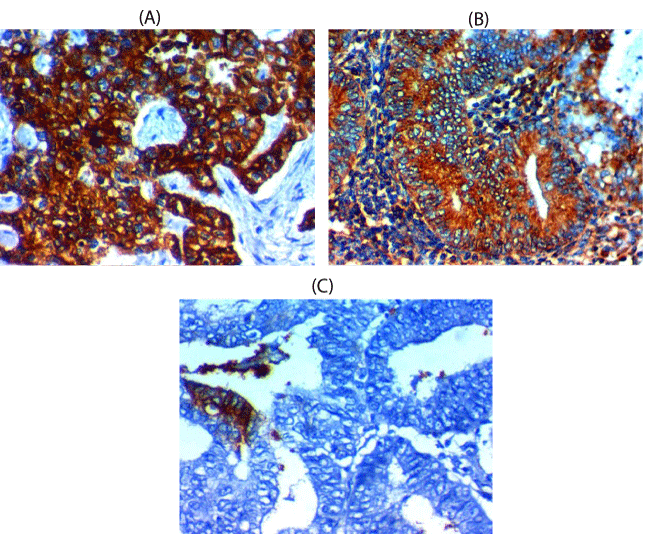
Nuclear expression was considered positive for SOX-2, while cytoplasmic and membranous expression was considered positive for L1CAM.
To score all the slides adequately and to reach a suitable final stain score we have combined scores of intensity and extent of the stain by multiplying both of them after examination of most fields of the tumor cells. The intensity was scored as followed (0, negative stain expression; 1, weak stain expression; 2, moderate stain expression and 3, strong stain expression and the extent was scored as followed (0 if stain expression in less than 1% of cancer cells; 1 if stain expression in 1-10% of tumor cells; 2 if stain expression in 10-25% of tumor cells; 3 if stain expression in 25-50% of tumor cells and 4 if stain expression in > 50% of tumor cells. Final immunoreactivity scores which resulted from multiplication of both intensity of stain and extent of stain scores ranged from 0-12, we have considered the value of 4 as a cutoff value above which is high expression and below which is low expression for adequate statistical analysis [14,15].
Statistical analysis
The collected data were computerized and statistically analyzed using SPSS program (Statistical Package for Social Science) version. Data were tested for normal distribution using the Shapiro Walk test.
Chi square and Fisher exact tests have been used to calculate differences among qualitative variables. P-value ≤ 0.05 indicates significant, p <0.001 indicates highly significant difference.
Spearman’s Rho Rank correlation test was used for correlating variables.
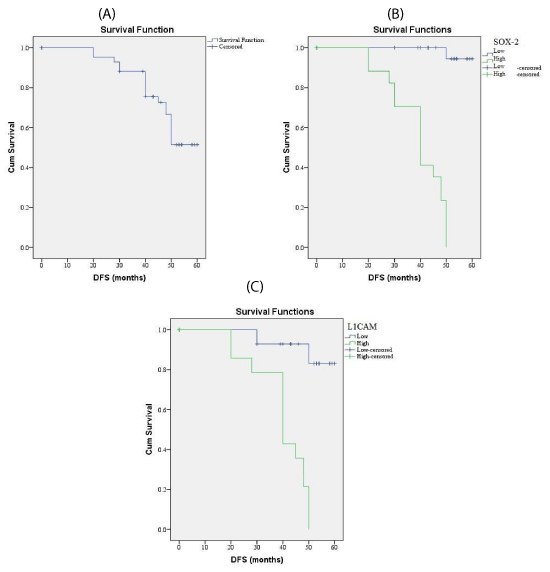
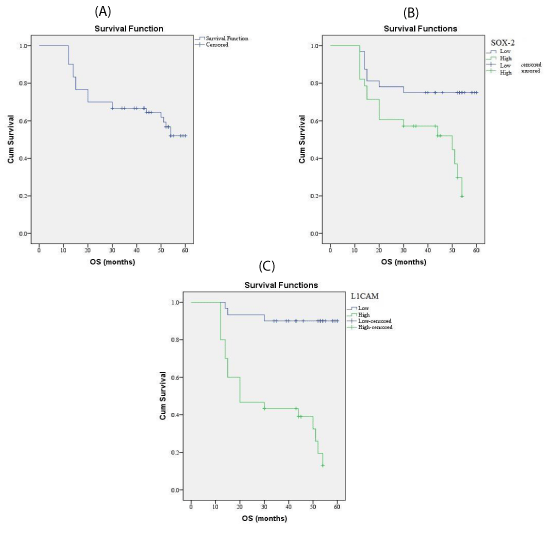
Survival analysis: Kaplan & Meier method has been used to estimate overall and disease free survival rates. Overall survival (OS) rate: was calculated as the time between the date of cancer diagnosis to death and last seen alive date.
Results
Patient clinical and pathological results
Clinical and pathological features of the included sixty patients with endometrioid carcinoma are detailed in table 1.
Immunohistochemical results
SOX-2 expression and association with clinical, pathological and follow-up findings tables 2,3, Figures 1,3,4
SOX-2 was overexpressed in 29 out of 60 (58%) cases of endometrioid carcinoma and it was associated with old age of the patient (p=0.003), larger tumor size(p=0.004), higher grade, advanced stage, presence of L.N and distant metastases (p<0.001), presence of myometrium invasion, cervical stromal invasion (p=0.006), lymphovascular (p=0.02)& parametril (p=0.007), serosa (p=0.03), adnexal invasions (p=0.008), shorter 5-year overall survival rate (p=0.003), and shorter 5-year disease free survival rate (p=0.005).
L1CAM expression and association with clinical, pathological and follow-up findings tables 2,4, Figures 2-4
L1CAM was overexpressed in 32 out of 60 (68%) cases of endometrioid carcinoma and it was associated with larger tumor size (p=0.017), higher grade (p=0.0 43),, advanced stage(p=0.0 31), presence of L.N (p=0.0 33), and distant metastases(p=0.0 49), presence of myometrium invasion (p=0.0 27), cervical stromal invasion (p=0.026), lymphovascular (p=0.02) & parametrial (p=0.012), serosa (p=0.042), adnexal invasions (p=0.034), shorter 5-year overall survival rate (p=0.002), and shorter 5-year disease free survival rate (p=0.006). There are no significant relations between L1CAM expression and age of the patient.
We found a direct relationship between SOX-2 and L1CAM expression in endometrioid carcinoma tissues; r (Correlation Coefficient) = +0.735 (P<0.001).
Discussion
The novel roles of SOX-2 as a transcription factor and stem cell marker are previously described to have many roles in cancer progression and metastasis in cancers of many organs [17]. More over increased SOX-2 expression is related to unfavorable clinco-pathological criteria and poorer outcomes of cancer patients [16-19]. There are still conflicting results regarding its role in progression and stem cells activation in endometrioid carcinoma of the uterus.
In this study, we found that high expression of SOX2 was positively correlated with unfavorable clinco-pathological criteria and poorer outcomes of cancer patients e.g. older age of the patient, higher grade, increased size, advanced stage of the tumor, presence of lymphatic and blood metastases, myometrial, lymphovascular, serosal, cervical and adnexal invasions. These results are similar to results of Pityński et al., who found a similar association between increased Sox-2 expression higher grade of endometrial carcinoma but they found no association between such expression and stage or presence of metastases as they performed their research on early stage endometrial carcinoma only [14], so a point of strength in our study is that we included all stages of endometrial carcinoma to detect association between its expression and stage of the tumor in our study, similarly, Yang et al., demonstrated that SOX-2 overexpression is related to higher grade, advanced stage and presence of lymph node metastases in patients with small cell lung cancer [20]. More over Neumann et al., proved that SOX2 expression was positively associated with higher incidence of nodal and distant metastasis in right-sided colon cancer [21]. Ruan et al., have proved results that are similar to ours that increased SOX-2 expression in bladder cancer was positively correlated with increased cancer size and grade [17]. Regarding the association of SOX-2 expression and higher grade of endometroid carcinoma there are many previous studies which have proved the same association in cancer cervix, breast, colon and lung and these studied explained their results by association of SOX-2 with cancer stem cells in many cancers [14,17].
All these results in addition to ours, clarified the essential role of SOX-2 in the carcinogenesis process which controls cancer cell proliferation potential.
We have proved that increased Sox 2 expression in endometrial carcinoma was related to older age of the patient which was slightly different from results of Wilbertz T et al. that showed increased SOX2 expression is associated with younger patient age in squamous cell lung cancer [22]. Yang et al., have proved that that increased Sox2 expression is a predictive biomarker in gastric cancer with earlier stage (Stages I & II), but they have not find that similar association with advanced stages (Stages III & IV) [23], but in the current study we have its prognostic roles in advanced and early stages of EMC. There are many explanations for the prognostic and predictive role of Sox-2 expression; SOX-2 is considered a transcription factor which has many roles in oncogenesis and cancer biology [24,25]. Due to different results regarding the prognostic roles of SOX-2, it was proved that it may have oncogenic or onco-suppressor roles according to type of cancer, Otsubo et al., explained the tumor suppressor role of SOX-2 by that it could inhibit of cell growth by inhibition of cyclin D1 and regulation of phosphorylated Rb [26]. SOX-2 might also activate PTEN directly [27]. Additionally, Sox2 could inhibit spread of cancer cells by increasing the expression of p21 [28].
The tumor initiating and oncogenic role of Sox2 was found in gastric cancer cells and blocking such role reduced invasion and metastases of gastric cancer cell [29], such oncogenic role is explained by that the aberrant expression of stem cell transcription factor; SOX-2 could impair the malignant stem cell like phenotype [30]. The cancer stem cells (CSCs) hypothesis affects the cancer management as so many novel targeted therapies depend on identification of CSCs specific bio-markers and that targeting these markers may be used as novel therapies in addition to the currently used therapies, to strengthen our findings we have assessed another adhesion biomarker which is L1CAM in endometrial carcinoma tissues and correlate it with the CSC marker SOX-2 to detect the possibility of using them as recent therapies for such type of cancer. We have found that there is increased the expression of L1CAM in endometrial carcinoma tissues and its expression was associated with poor tumor differentiation, metastatic disease in addition to association with poor response to therapy and unfavorable survival rates which clarified the poor prognostic effect of such biomarker and our results were similar to those of Kommoss et al., who have assessed the prognostic role of L1CAM expression in patients with endometroid carcinoma and find a similar association between L1CAM expression and poor prognostic parameters and higher risk group which proved that L1CAM allow better risk stratification of patients [31]. Results of the current study is similar to results of previous studies Smogeli et al. that have reported that increased L1CAM expression in endometrial carcinoma is related to unfavorable outcome [32]. and other poor prognostic parameters e.g. non-endometrioid histopathology, LVSI and high grade of the tumor and also our results are similar to results of many previous studies about the prognostic role of L1CAM expression in cancer [33-35], similarly Zeimet et al. proved that L1CAM is a marker of cancer prognosis and increased its expression has been associate with a poor outcome of patients with endometroid carcinoma [35]. Moreover, our results regarding the association of L1CAM expression and unfavorable prognosis in endometroid carcinoma was similar to results of previous studies [37,38]. Endometroid carcinoma is usually a diseases of favorable prognosis and previous studies found that the cause of poor prognosis of certain cases of endometroid carcinoma is the presence of non-endometroid foci in them Geels et al., which was in line with our results that we have found positive association between L1CAM expression and presence of non-endometroid foci in endometroid carcinoma [34]. In the current study we have proved that high L1CAM expression was associated with dismal 5-year survival rates and with recurrent tumor after successive therapy that was also similar to Geels et al. [34].
Similarly, Sagae et al., have detect the association of L1CAM expression and presence of non-endometrioid foci and it was increased in non-endometroid carcinoma serous subtype II carcinomas, and they have proved that L1CAM overexpression was associated with poor clinical, pathological criteria and patient outcome [39].
L1CAM has many actions that explained its association with aggressive behavior and more invasive pattern of malignancies that showed increased tissue protein expression of such marker through increasing malignant cell invasion and motility via activation of Wnt signaling pathway which could be able to stimulate the epithelial mesenchyme transition (EMT) process which is incriminated in cancer metastases [36], and that it could act as a pro-angiogenic factor which is responsible for neo-angiogenesis in cancer tissues that increased its invasion and metastases [40].
The role of L1CAM in neo-angiogenesis in cancer tissues and invasion of the blood vessels that explained its role as a poor prognostic marker could be explained by Kommoss et al., Geels et al. 2016 and Putten et al. 2016 results who found positive correlation between L1CAM and lymphovascular and stromal invasion (LVSI) [31,34,35].
These results clarified the values of adding LVSI in recent ESMO risk stratification guidelines, and highlight the need for evaluation of LVSI as an important parameter during histopathological evaluations of endometrial carcinoma. We have found that high L1CAM expression is associated with higher incidence of recurrence after successive therapy which was similar to results of Kommoss et al., and Colombo et al. [31,41]. So, it will be beneficial to predict recurrence in endometrial carcinoma cases with increased L1CAM expression.
Additionally our data and results of previous studies regarding the association between increased L1CAM expression, poor clinicopathological features and dismal patients outcome, suggested that those patients may benefit from targeted therapies against mediated L1CAM [42].
Previous studies clarified L1CAM role in EMT by activation of beta-catenin/TCF pathway in plethora cancers of different organs [15,43,44]. Moreover, L1CAM has been linked to the cancer stem cell (CSC) theory and identified as CSC marker [45], Bao et al., explained that by expression of L1CAM in glioma cells with high CD133 expression that is a CSC marker [36].
To clarify its role as a CSC marker we correlate its expression withSOX-2 that is a stem cell biomarker in endometrial carcinoma tissue and we have find a significant positive association between both markers expression and that overexpression of both of them were associated with poor clinicopathological and prognostic findings which suggested that molecular targeted therapy against them could be used as novel therapies for endometrial carcinoma.
In summary, we have hypothesized that both Sox-2 and L1CAM expressions might be able to induce CSCs activation and stimulate EMT in endometroid carcinoma cells which facilitate, progression, invasion and metastases, so targeted therapies against them might be novel promising therapies which could improve patients prognosis in addition to the currently used therapies.
In conclusion, increased Sox-2 and L1CAM expression are markers of poor prognosis and dismal outcome of patients with endometrioid carcinoma of the uterus.
- rennan DJ, Hackethal A, Metcalf AM, Coward J, Ferguson K, et al. (2014) “SerumHE4 as a prognosticmarker in endometrial cancer—population based study,” Gynecologic Oncology 1: 159–165. Link: https://tinyurl.com/yb42yu2p
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. Link: https://tinyurl.com/yaheq59e
- Morice P, Leary A, Creutzberg C, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. The Lancet 387: 1094–1108. Link: https://tinyurl.com/y7j8pk2q
- Engelsen IB, Akslen LA, Salvesen HB. Biologic markers in endometrial cancer treatment. APMIS 2009; 117(10):693–707. Link: https://tinyurl.com/y9gqrvxu
- Hubbard SA, Friel AM, Kumar B, Zhang L, Rueda BR, et al. (2009) Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res 69: 8241–8248. Link: https://tinyurl.com/y9ycwtow
- Mirantes C, Espinosa I, Ferrer I, Dolcet X, Prat J, et al. (2013) Epithelial-tomesenchymal transition and stem cells in endometrial cancer. Hum Pathol 44: 1973–1981. Link: https://tinyurl.com/yd44zuqc
- Skidan I, Steiniger SC (2014) In vivo models for cancer stem cell research: a practical guide for frequently used animal models and available biomarkers. J Physiol Pharmacol 65: 157-169. Link: https://tinyurl.com/yd2mwprz
- Niwa H (2007) how is pluripotency determined and maintained? Development 134: 635-646. Link: https://tinyurl.com/y8o3kvg5
- Sarkar A, Hochedlinger K (2013) The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12: 15-30. Link: https://tinyurl.com/yd5bhduz
- Bessede E, Staedel C, Amador LAA, Nguyen PH, Chambonnier L, et al. (2013) Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene. Link: https://tinyurl.com/y7d3bqzt
- Bondong S, Kiefel H, Hielscher T, Zeimet AG, Zeillinger R, et al. (2012) Prognostic significance of L1CAM in ovarian cancer and its role in constitutive NF-kappaB activation. Ann Oncol 23: 1795–1802. Link: https://tinyurl.com/y8xpoywh
- Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, et al. (2010) Comparative performance of the 2009 international federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet Gynecol 116: 1141–1149. Link: https://tinyurl.com/y76fogzl
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J HistochemCytochem 29: 577-580. Link: https://tinyurl.com/ycaqbvsl
- Pityński K, Banas T, Pietrus M, Milian-Ciesielska K, Ludwin A, et al. (2015) SOX-2, but not Oct4, is highly expressed in early-stage endometrial adenocarcinoma and is related to tumour grading Int J Clin Exp Pathol 8: 8189-8198. Link: https://tinyurl.com/y7zawrrz
- Guo X, Xiong L, Zou L, Sun T, Zhang J, et al. (2012) L1 cell adhesion molecule overexpression in hepatocellular carcinoma associates with advanced tumor progression and poor patient survival Guo et al. Diagnostic Pathology 7: 96. Link: https://tinyurl.com/ybevexgl
- Lin CW, Liao MY, Lin WW, Wang YP, Lu TY, et al. (2012) Epithelial cell adhesion molecule regulates tumor initiation and tumorigenesis via activating reprogramming factors and epithelial-mesenchymal transition gene expression in colon cancer. J Biol Chem 287: 39449-39459. Link: https://tinyurl.com/yclta2na
- Ruan J, Wei B, Xu Z, Yang S, Zhou Y, et al. (2013) Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med Oncol 30: 445. Link: https://tinyurl.com/y8bnqmwh
- Ji J, Wei X, Wang Y (2014) Embryonic stem cell markers Sox-2 and OCT4 expression and their correlation with WNT signal pathway in cervical squamous cell carcinoma. Int J Clin Exp Pathol 7: 2470-2476. Link: https://tinyurl.com/yau6lfn2
- Wang Q, He W, Lu C, Wang Z, Wang J, et al. (2009) Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human oesophageal squamous cell carcinoma. Anticancer Res 29: 1233-1241. Link: https://tinyurl.com/y84qfe9v
- Yang F, Gao Y, Geng J, Qu D, Han Q, et al. (2013) Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol 6: 2846-2854. Link: https://tinyurl.com/y7e2vop4
- Neumann J, Bahr F, Horst D, Kriegl L, Engel J, et al. (2011) SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer 11: 518. Link: https://tinyurl.com/ybdtnpfn
- Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, et al. (2011) SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Mod Pathol 24: 944- 953. Link: https://tinyurl.com/y8m8hxe6
- Yang L, Xu J, Kang Q, Ai-Qin Li, Jin P, et al. (2017) Predictive Value of Stemness Factor Sox2 in Gastric Cancer Is Associated with Tumor Location and Stage. PLOS ONE. Link: https://tinyurl.com/y9zefpn3
- Weina K, Utikal J (2014) SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med 3: 19. Link: https://tinyurl.com/y9ryozhb
- Carrasco-Garcia E, Santos JC, Garcia I, Brianti M, García-Puga M, et al. (2016) Paradoxical role of SOX2 in gastric cancer. Am J Cancer Res 6: 701–713. Link: https://tinyurl.com/yc6j9pv5
- Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y (2008) SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer 98: 824-831. Link: https://tinyurl.com/y7opk9ac
- Wang S, Tie J, Wang R, Hu F, Gao L, et al. (2015) SOX2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating PTEN. Cancer Lett 358: 210-219. Link: https://tinyurl.com/y8aczsuy
- Chen Y, Huang Y, Zhu L, Chen M, Huang Y, et al. (2016) SOX2 inhibits metastasis in gastric cancer. J Cancer Res Clin Oncol 142: 1221-1230. Link: https://tinyurl.com/ycdum8bb
- Hutz K, Mejias-Luque R, Farsakova K, Ogris M, Krebs S, et al. (2014) The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis 35: 942-950. Link: https://tinyurl.com/ydckeoae
- Shen JX, Liu J, Li GW, Huang YJ, Wu HT, et al. (2016) Mining distinct aldehyde dehydrogenase 1 (ALDH1) isoenzymes in gastric cancer. Oncotarget. Link: https://tinyurl.com/y7d43ex2
- Kommoss F, Kommoss F,Grevenkamp F, Bunz AK, Taran FA, et al. (2017) L1CAM: amending the “low‑risk” category in endometrial carcinoma J Cancer Res Clin Oncol 143: 255–262. Link: https://tinyurl.com/y99ox7ec
- Smogeli E , Davidson B, Cvancarova M, Holth A, Katz B, et al. (2016) L1CAM as a prognostic marker in stage I endometrial cancer: a validation study. BMC Cancer 16: 596. Link: https://tinyurl.com/y9sx66us
- Bosse T, Nout RA, Stelloo E, Dreef E, Nijman HW, et al. (2014) L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: pooled PORTEC trial results. Eur J Cancer 50: 2602–2610. Link: https://tinyurl.com/y9k3bldu
- Geels YP, Pijnenborg JA, Gordon BM, Fogel M, Altevogt P, et al. (2016) L1CAM expression is related to non-endometrioid histology, and prognostic for poor outcome in endometrioid endometrial carcinoma. Pathol Oncol Res. Link: https://tinyurl.com/yae5r6cy
- Putten VD, Visser NC, Vijver KV, Santacana M, Bronsert P, et al. (2016) L1CAM expression in endometrial carcinomas: an ENITEC collaboration study. Br J Cancer. Link: https://tinyurl.com/y7upoxz3
- Zeimet AG, Reimer D, Huszar M, Winterhoff B, Puistola U, et al. (2013) L1CAM in early-stage type I endometrial cancer: results of a large multicenter evaluation. Inst 105: 1142–1150. Link: https://tinyurl.com/y7z325x8
- Van Gool IC, Stelloo E, Nout RA, Nijman HW, Edmondson RJ, et al. (2016) Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer Modern Pathology 29: 174–181. Link: https://tinyurl.com/ycx74gfl
- Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497:67–73. Link: https://tinyurl.com/ybkoedk5
- Sagae S, Susumu N, Viswanathan AN, Aoki D, Backes FJ, et al (2014) Gynecologic Cancer InterGroup (GCIG) consensus review for uterine serous carcinoma. Int J Gynecol Cancer off J Int Gynecol Cancer Soc 24: 83–89. Link: https://tinyurl.com/ybsfvtru
- Kiefel H, Bondong S, Hazin J, Ridinger J, Schirmer U, et al. (2012) L1CAM: a major driver for tumor cell invasion and motility. Cell Adhesion Migrat 6: 374–384. Link: https://tinyurl.com/y7zmdexy
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, et al. (2016) ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. Link: https://tinyurl.com/y7xaocd9
- Doberstein K, Harter PN, Haberkorn U, Bretz NP, Arnold B, et al. (2015) Antibody therapy to human L1CAM in a transgenic mouse model blocks local tumor growth but induces EMT. Int J Cancer 136: 326–339. Link: https://tinyurl.com/y7txftrw
- Tischler V, Pfeifer M, Hausladen S, Schirmer U, Bonde AK, et al. (2011) L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Molecular Cancer 10:127. Link: https://tinyurl.com/ydcjkckb
- Gavert N, Conacci-Sorrell M, Gast D, et al. (2005) L1, a novel target of beta-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol 168: 633-642. Link: https://tinyurl.com/ybe2yors
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133: 704-715. Link: https://tinyurl.com/y74nvtdx
- Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, et al. (2008) targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res 68: 6043-6048. Link: https://tinyurl.com/yddh4xvt
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
