Annals of Cytology and Pathology
Development of a management guide wheel for cervical cancer
Mahmoud Samy Ismail1-4*, Fouad Ismail5, Julia Ismail6, Muneera AlKhalifa7, Rehab Ismael1, Reham Fathi1, Wassan Al Ani1, Shaikha Al Hajri1,Nusiba Elhassan1, Mooza AlKawari1, Aysha AlBinali1, Gulmeen Raza1, Mariam Fida12, Alaa Zeineldine1 and Uwe Torsten3,4,8
2Bahrain Oncology Centre (BOC), Kingdom of Bahrain
3Department of Gyne-Oncology, Charitè University Hospital, Humboldt University, Berlin, Germany
4Pan Arabian Research Society of Gynecological Oncology (PARSGO), Kingdom of Bahrain and Berlin, Germany
5University of Applied Arts, Vienna, Austria
6Vienna University of Technology, Vienna, Austria
7King Hamad University Hospital (KHUH), Kingdom of Bahrain
8Head of Department of Obstetrics and Gynecology, Royal College of Surgeons in Ireland (RCSI), Kingdom of Bahrain
Cite this as
Ismail MS, Ismail F, Ismail J, AlKhalifa M, Ismael R, et al. (2022) Development of a management guide wheel for cervical cancer. Ann Cytol Pathol 7(1): 014-028. DOI: 10.17352/acp.000025Copyright License
© 2022 Ismail MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.We designed and developed a management guide wheel for cervical cancer to facilitate a standard approach and improve the quality of care in managing patients diagnosed with different stages of cervical cancer. Each step of the wheel considers the patient’s current medical condition, FIGO stage, and the possible treatment modalities for cervical cancer in her case. We reviewed existing international guidelines on the management of cervical cancer and compared their respective recommendations. This guide wheel is based on recommendations by the following organizations: the National Comprehensive Cancer Network (NCCN), the Society of Gynecologic Oncology (SGO), the European Society of Gynaecological Oncology (ESGO), the European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), the British Gynaecological Cancer Society (BGCS) and the International Federation of Gynecology and Obstetrics (FIGO).
Abbreviations
ASCO: American Society of Clinical Oncology; BGCS: British Gynaecological Cancer Society; CC: cervical cancer; CT: Computed Tomography; EBRT: External Beam Radiotherapy; ESGO: European Society of Gynaecological Oncology; ESMO: European Society for Medical Oncology; FIGO: International Federation of Gynecology and Obstetrics; IORT: Intra-Operative Radiotherapy; LN: Lymph Node; LND: Lymph Node Dissection; LVSI: Lymphovascular Space Invasion; MRI: Magnetic Resonance Imaging; NCCN: National Comprehensive Cancer Network; PET: Positron Emission Tomography; RH: Radical Hysterectomy; SGO: Society of Gynecologic Oncology; TNM: Tumors, Nodes, and Metastases.
Introduction
Cervical Cancer (CC) is the fourth most common cancer in women worldwide and the second most common cancer in developing countries [1-7]. The mainstay of treating CC involves surgery, radiotherapy, chemotherapy, or a combination of those [6-9]. Early-stage CC is usually asymptomatic and patients present with symptoms in advanced stages [4,5,10]. CC can be preventable with effective screening and vaccination programs [4,5,11,12]. Early detection of pre-malignant states before they develop into cancer leads to earlier treatment and hence, a better outcome [12]. In fact, early-stage CC has a cure rate of 80-95% [8]. 30% of CC in developed countries are Stage IB or higher compared to 60% in developing countries due to the unavailability of well-established screening programs in the latter [2,12].
Various health organizations globally have developed sets of guidelines on the management of CC. We compared recommendations by the National Comprehensive Cancer Network (NCCN), the Society of Gynecology (SGO), the European Society of Gynaecological Oncology (ESGO), the European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), the British Gynaecological Cancer Society (BGCS) and the International Federation of Gynecology and Obstetrics (FIGO) [4-6,9,10,13,14]. The NCCN guidelines for managing cancer cervix is one of the most frequently updated and comprehensive guidelines available [4].
We developed a management guide wheel for CC based on the recommendations of the compared guidelines but mainly reflecting the most recently updated guidelines by NCCN. This guide wheel aims to serve healthcare professionals in finding a quick and comprehensive display of the recommended steps and alternatives to manage patients diagnosed with CC when counseling patients. We are also comparing the guidelines covered here in another research article to highlight the similarities and differences in their respective recommendations. We developed a similar guide wheel for the management of uterine neoplasms [15]. We also developed a guide wheel describing the management of abnormal pap smear results and a comparison of the recommendations of international organizations [12,16].
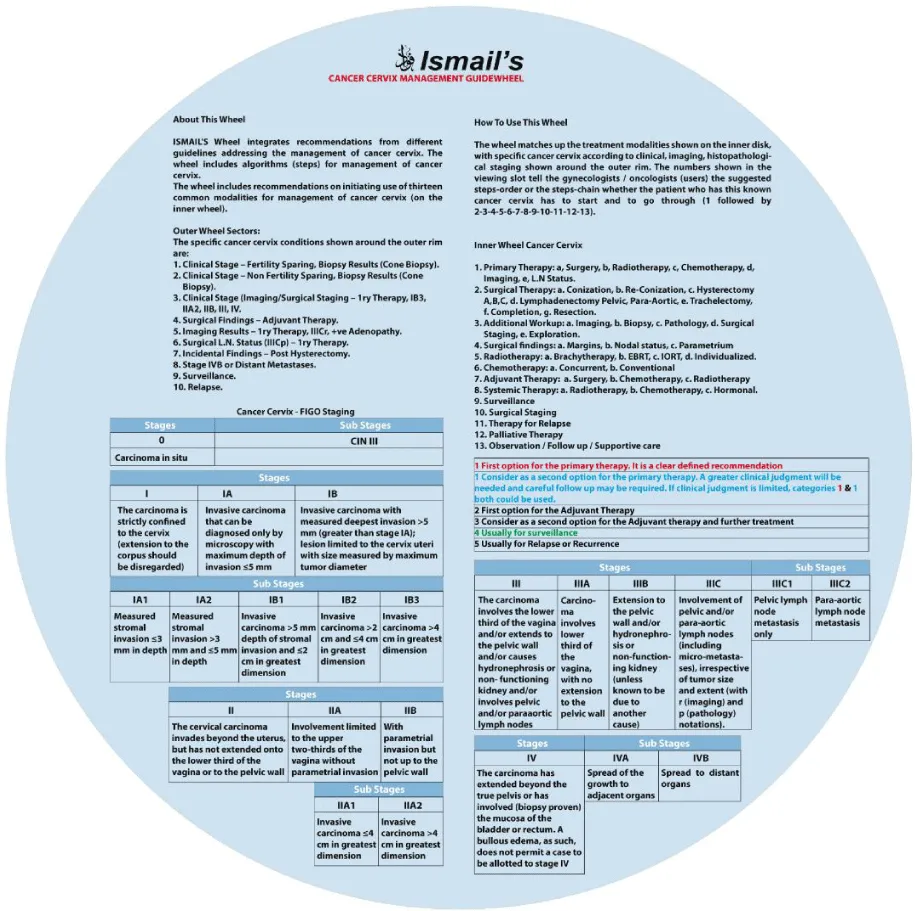
About this wheel
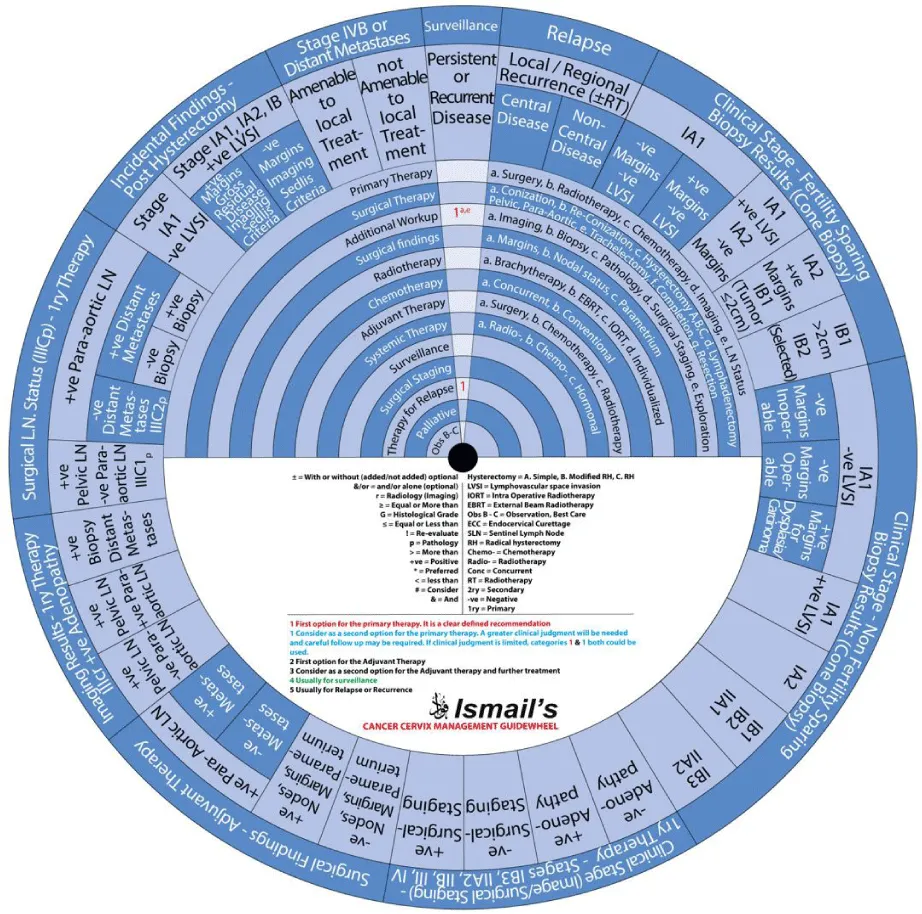
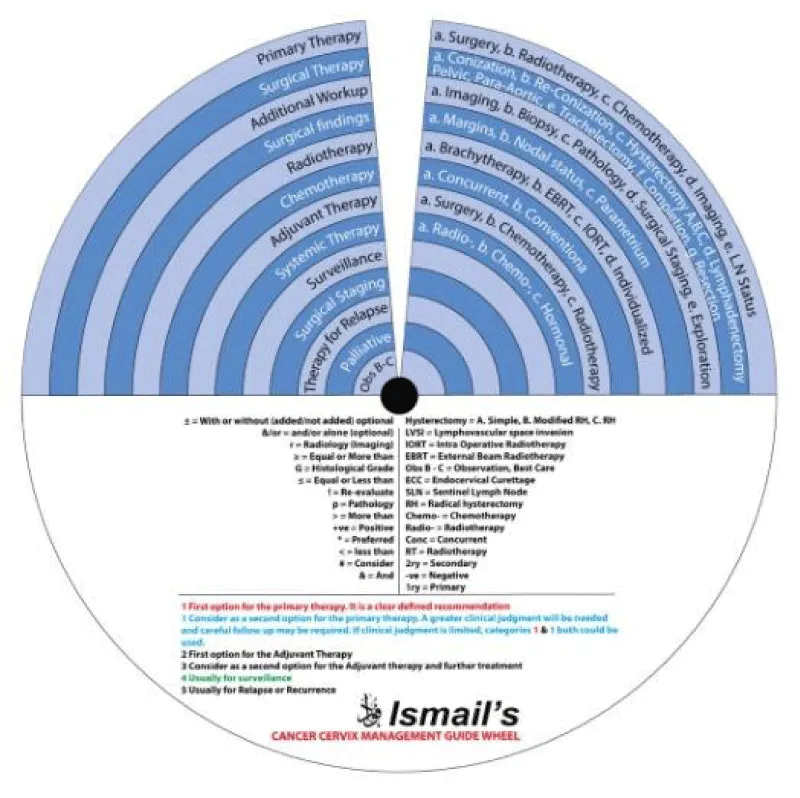
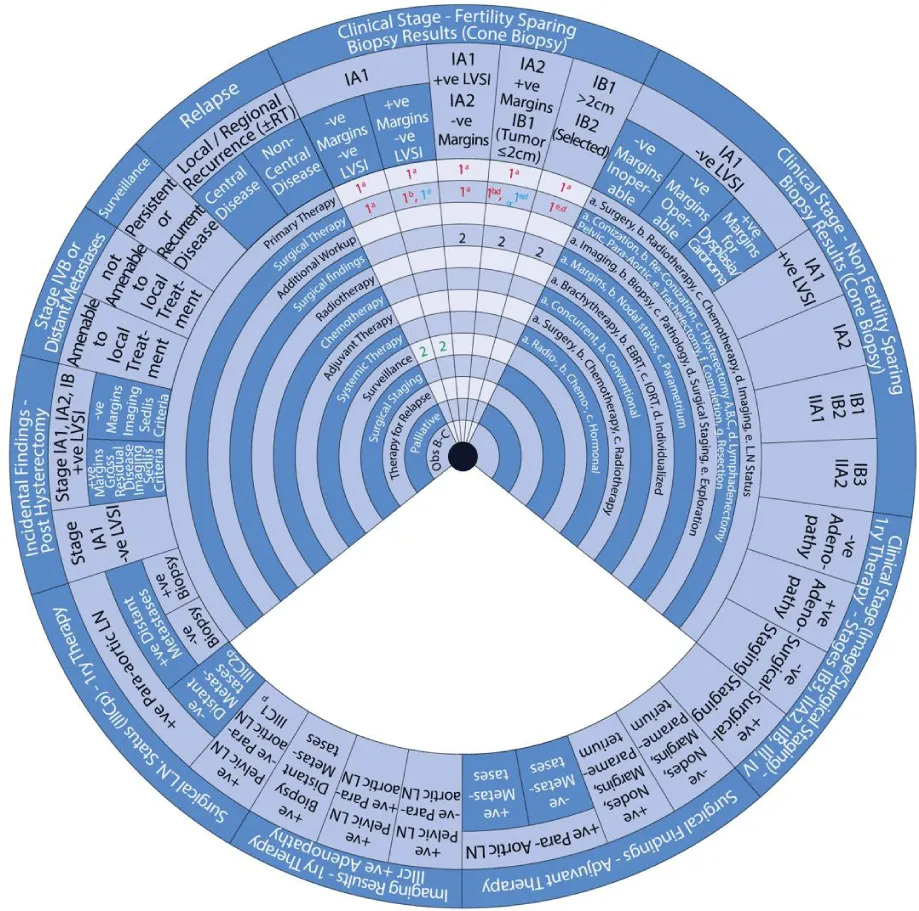
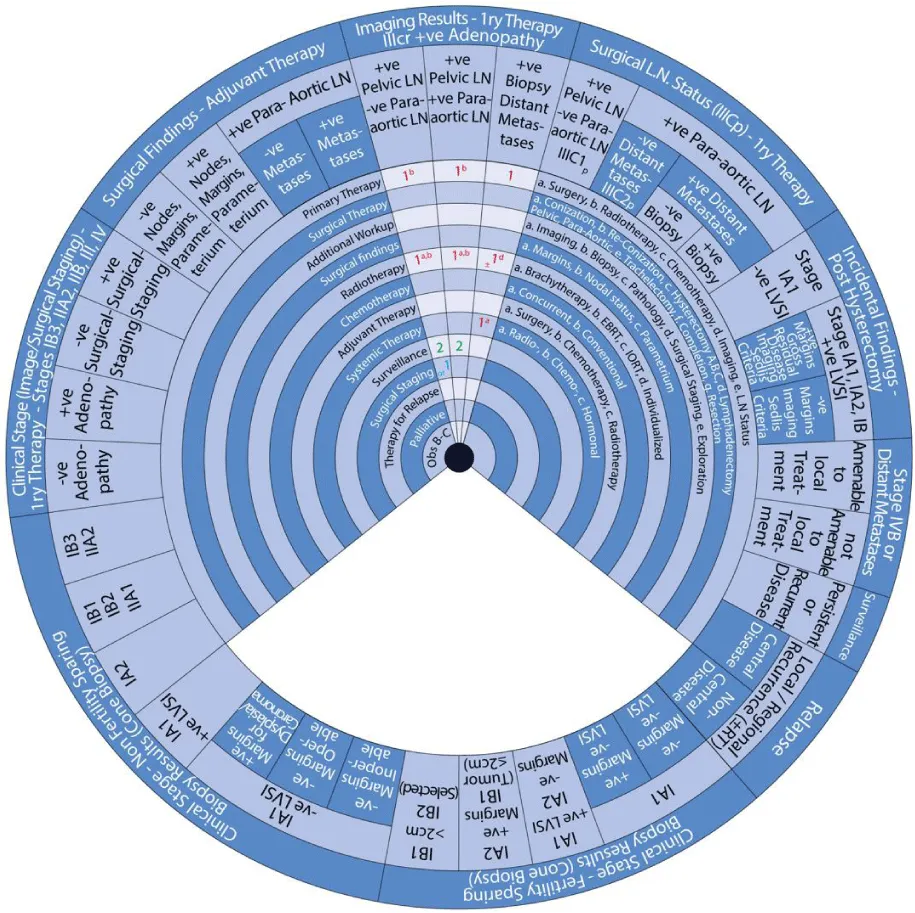
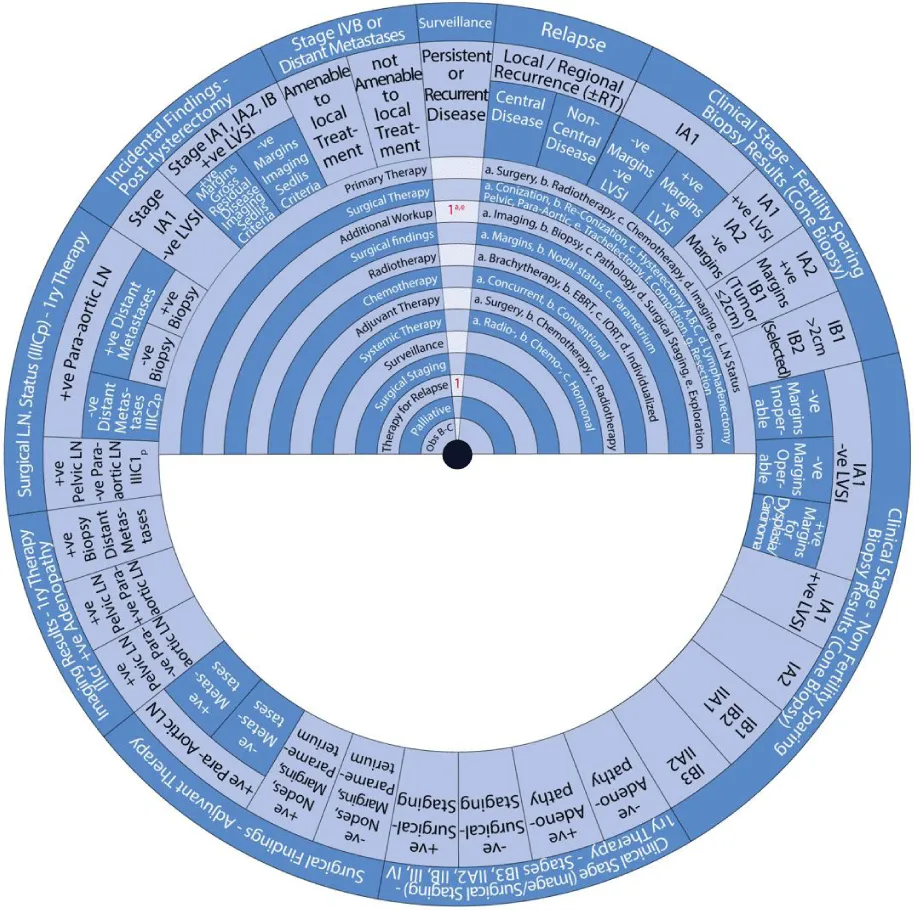
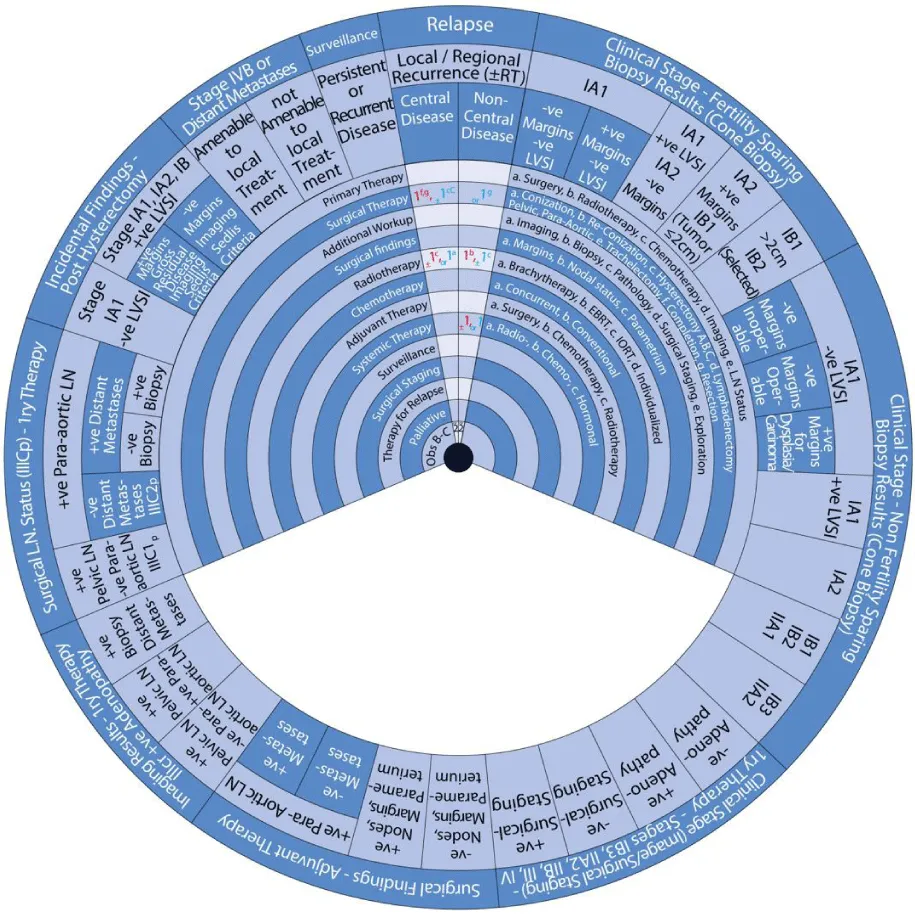
This guide wheel includes recommendations and algorithms for managing patients with CC as per the guidelines compared during this study (Figure 1). It consists of a base (Figure 2) and an inner wheel (Figure 3). There is more information on how to use the guide wheel on the back of the base (Figure 4).
There are thirteen possible modalities of treatment with the different options under those modalities mentioned on the inner wheel as follows:
- Primary Therapy: a. Surgery; b. Radiotherapy; c. Chemotherapy; d. Imaging; e. Lymph node (LN) status
- Surgical Therapy: a. Conization; b. Re-Conization; c. Hysterectomy (A. Simple; B. Modified Radical Hysterectomy (RH); C. RH); d. Lymphadenectomy (Pelvic; Para-aortic); e. Trachelectomy; f. Completion; g. Resection
- Additional Workup: a. Imaging; b. Biopsy; c. Pathology; d. Surgical Staging; e. Exploration
- Surgical findings: a. Margins; b. Nodal status; c. Parametrium
- Radiotherapy: a. Brachytherapy; b. External Beam Radiotherapy (EBRT); c. Intraoperative Radiotherapy (IORT); d. Individualized
- Chemotherapy: a. Concurrent; b. Conventional
- Adjuvant Therapy: a. Surgery; b. Chemotherapy; c. Radiotherapy
- Systemic Therapy: a. Radiotherapy; b. Chemotherapy; c. Hormonal
- Surveillance
- Surgical Staging
- Therapy for Relapse
- Palliative Therapy
- Observation, Follow-up and Supportive care.
How to use the guidewheel
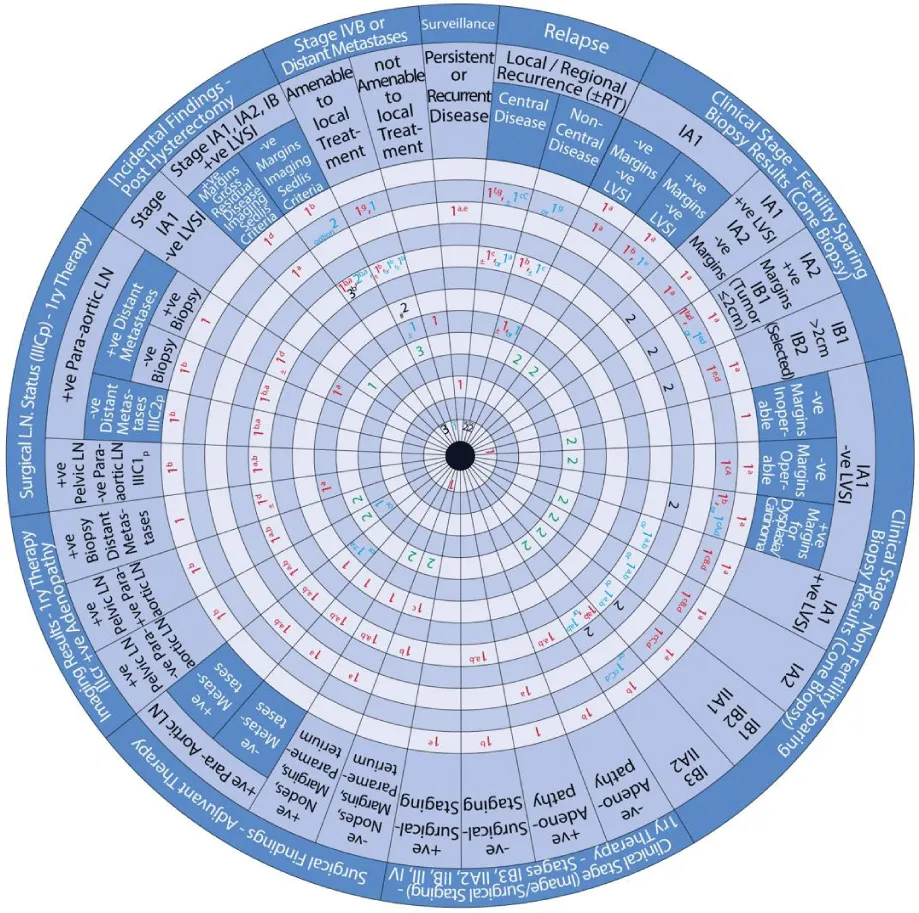
The guide wheel aligns the treatment modalities on the inner wheel with the particular stage of cervical cancer diagnosed, on the outer wheel, following clinical, radiological, and histopathological investigations. The viewing slot displays the steps recommended to follow in chronological order to manage the patient under consideration (Figure 1).
The base of the guide wheel displays color-coded treatment modalities in order. The numbers signify the steps and alternative options as follows:
1 → This marks the first option for Primary Therapy. It is a clearly defined recommendation.
1 → This is a second option for Primary Therapy. It might require greater clinical judgment and careful follow-up. If the clinical judgment is limited, either option 1 or 1 could be used.
2 → This marks the first option for Adjuvant Therapy.
3 → This is considered as a second option for Adjuvant Therapy and further treatment.
4 → This is usually reserved for surveillance.
5 → This is usually reserved for Relapse or Recurrence.
Abbreviations used on the wheel include the following:
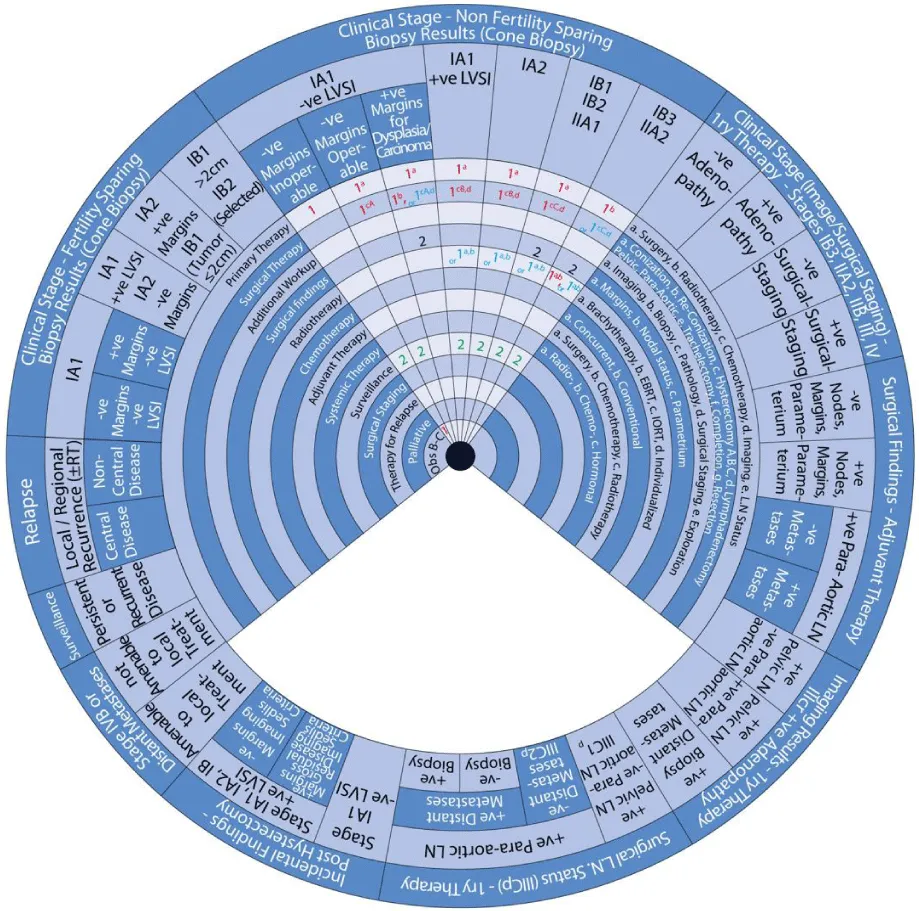
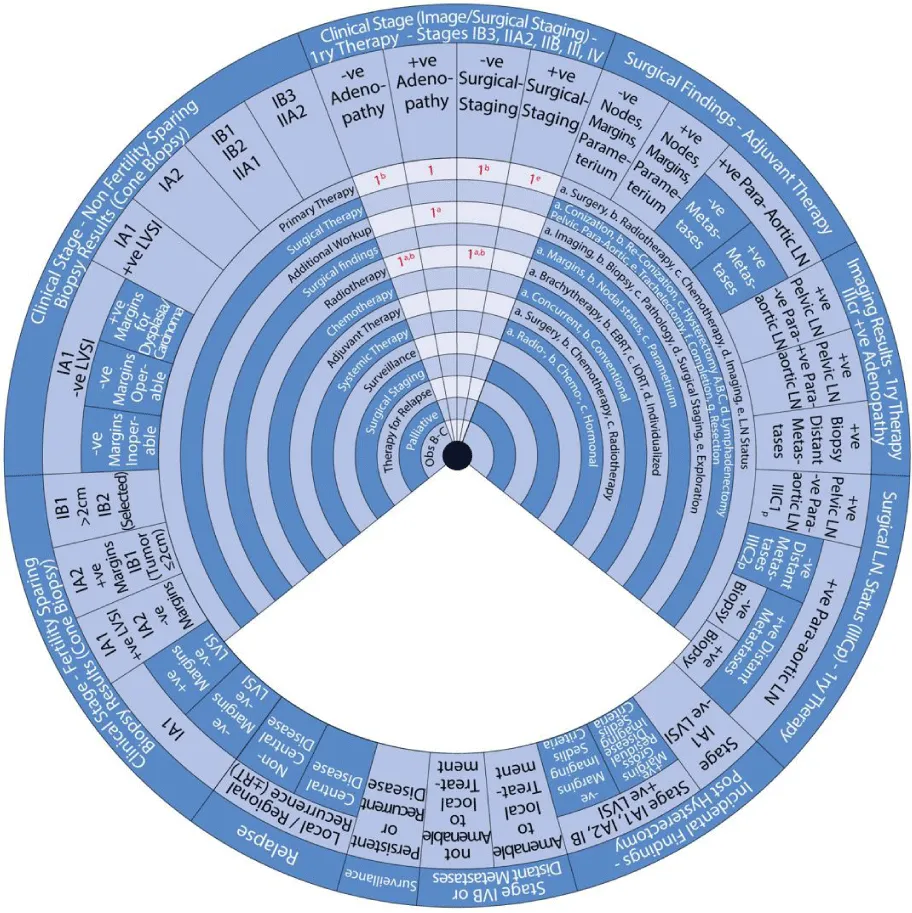
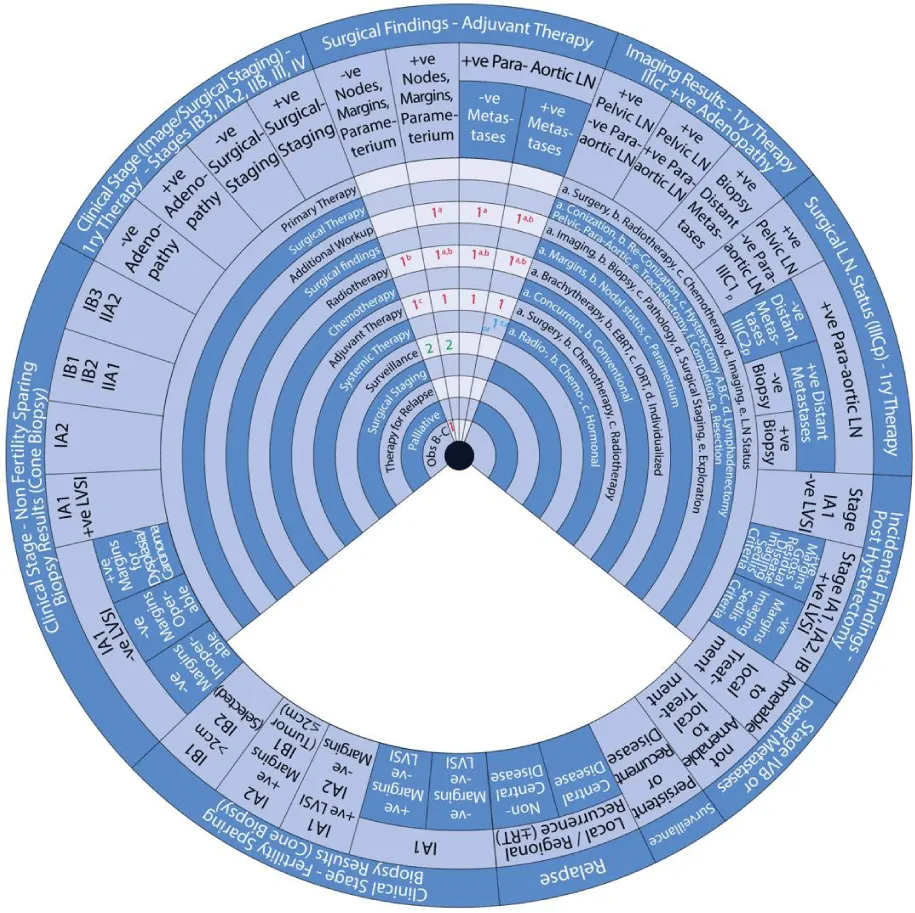
The stages of cervical cancer shown on the outer wheel are as follows:
- Fertility-Sparing Options for Stages IA1, IA2, IB1, and select IB2 Based on Cone Biopsy Results
- Non-Fertility Sparing Options for Stages IA1, IA2, IB1, IB2, IB3, IIA1, and IIA2 Based on Cone Biopsy Results
- Primary Therapy for Stages IB3, IIA2, IIB, III, and IV Based on Imaging or Surgical Staging
- Adjuvant Therapy Based on Surgical Findings
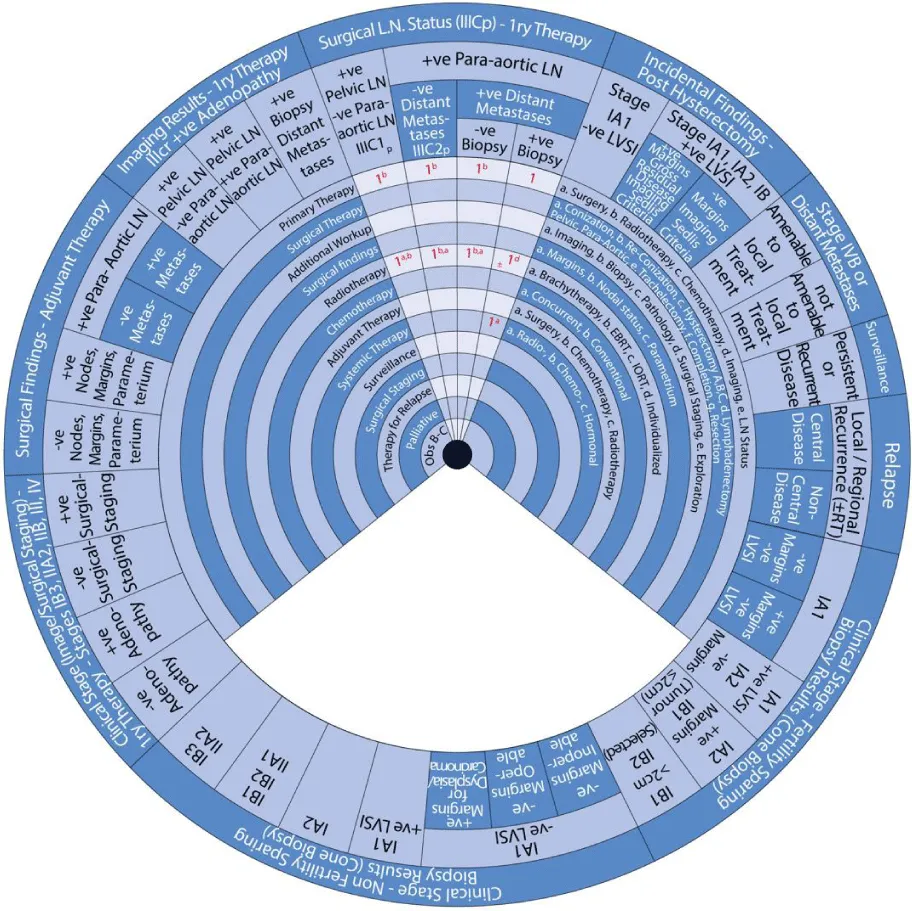
- Primary Therapy Based on Imaging Results Demonstrating Positive Adenopathy
- Primary Therapy Based on Surgical Lymph Node Status
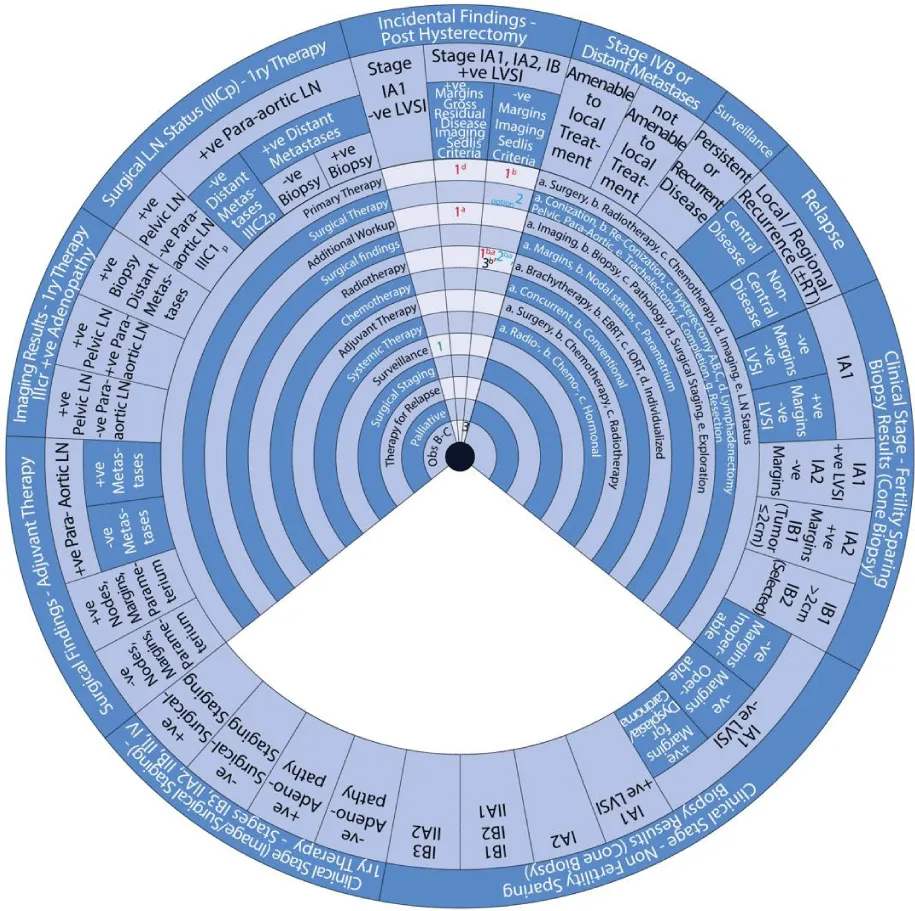
- Incidental Surgical Finding of Cervical Cancer Post-Hysterectomy
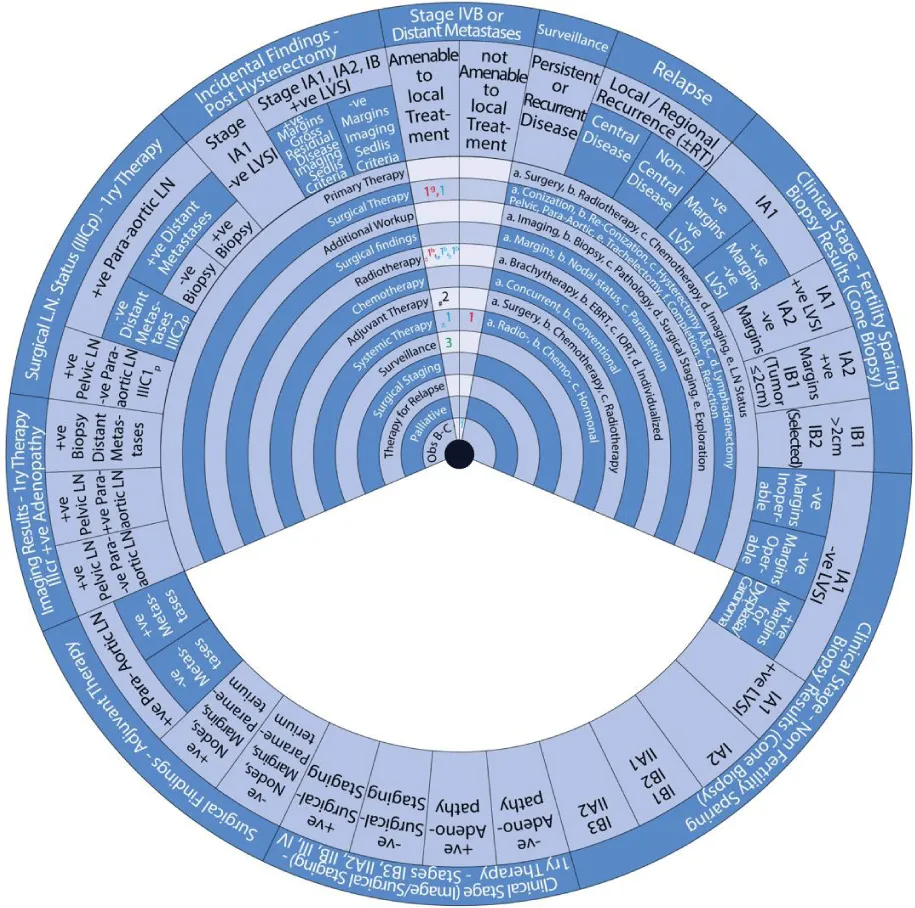
- Stage IVB or Distant Metastases
- Surveillance of Persistent or Recurrent Disease
- Therapy for Relapse
The back of the wheel displays the FIGO staging system for cervical cancer, most recently updated in 2018, as followed by the international guidelines. The table below exhibits the stages of cervical cancer and their definitions [1, 4, 6, 7, 9, 10].
Recommendations on managing patients with cervical cancer using the guide wheel
Sector 1: Fertility-Sparing Options for Stages IA1, IA2, IB1, and select IB2 Based on Cone Biopsy Results (Figure 5)
For patients with stage IA1 cervical cancer with negative margins and negative LVSI:
- 1a: Primary treatment is surgery (1a).
- 1a: Surgical treatment (1) with conization (a).
- 2: The second step is surveillance.
For patients with stage IA1 cervical cancer with positive margins and negative LVSI:
- 1a: Primary treatment is surgery (1a).
- 1b: Surgical treatment (1) with re-conization (b).
- 1e: An alternative is a surgical treatment (1) with trachelectomy (e).
- 2: The second step is surveillance.
For patients with stage IA1 cervical cancer with positive LVSI and stage IA2 with negative margins:
- 1a: Primary treatment is surgery (1a).
- 1e, d: Surgical treatment (1) with conization (e) with pelvic lymphadenectomy with or without para-aortic lymphadenectomy (d). Sentinel LN mapping can be considered.
- or 1a, d: An alternative is a surgical treatment (1) with conization (a) with pelvic lymphadenectomy. Sentinel LN mapping can also be considered if negative margins.
- 2: The second step depends on surgical findings.
For patients with stage IA2 cervical cancer with positive margins and stage IB1 with tumor measuring ≤ 2 cm:
- 1a: Primary treatment is surgery (1a).
- 1e, d: Surgical treatment (1) with conization (e) with pelvic lymphadenectomy with or without para-aortic lymphadenectomy (d). Sentinel LN mapping can be considered.
- or 1b, d: An alternative is surgical treatment (1) with re-conization (b) with pelvic lymphadenectomy. Sentinel LN mapping can also be considered.
- 2: The second step depends on surgical findings.
For patients with stage IB1 with a tumor measuring > 2 cm and select cases of stage IB2 ≤ 2 cm:
- 1a: Primary treatment is surgery (1a).
- 1e, d: Surgical treatment (1) with radical trachelectomy (e) with pelvic lymphadenectomy with or without para-aortic lymphadenectomy (d). Sentinel LN mapping can be considered.
- 2: The second step depends on surgical findings. This does not apply to patients with small neuroendocrine or gastric type adenocarcinoma of adenoma malignum.
Sector 2: Non-Fertility Sparing Options for Stages IA1, IA2, IB1, IB2, IB3, IIA1 and IIA2 Based on Cone Biopsy Results (Figure 6)
For patients with an inoperable stage IA1 cervical cancer with negative margins and negative LVSI:
- 1: The first step is observation (1).
- 2: The second step is surveillance.
For patients with an operable stage IA1 cervical cancer with negative margins and negative LVSI:
- 1a: Primary treatment is surgery (1a).
- 1cA: Surgical treatment (1) with simple hysterectomy (cA).
- 2: The second step is surveillance.
For patients with stage IA1 cervical cancer with margins positive for dysplasia or carcinoma but negative LVSI:
- 1a: Primary treatment is surgery (1a).
- 1b: Surgical treatment (1) with re-conization (b) with pathological re-evaluation.
- or 1cA, d: An alternative is surgical treatment (1) with simple hysterectomy (cA) or modified RH with pelvic lymphadenectomy (d). Sentinel LN mapping can also be considered.
- 2: The second step depends on surgical findings.
For patients with stage IA1 cervical cancer with positive LVSI and Stage IA2:
- 1a: Primary treatment is surgery (1a).
- 1cB, d: Surgical treatment (1) with modified RH (cB) with pelvic lymphadenectomy (d). Sentinel LN mapping can also be considered.
- or 1a, b: An alternative is a radiotherapy (1) with EBRT (b) and brachytherapy (a).
- 2: The second step is surveillance.
For patients with cervical cancer stages IB1, IB2, and IIA1:
- 1a: Primary treatment is surgery (1a).
- 1cC, d: Surgical treatment (1) with RH (cB) and pelvic lymphadenectomy (d) with or without para-aortic lymphadenectomy. Sentinel LN mapping can also be considered.
- or 1a, b: An alternative is a radiotherapy (1) with EBRT (b) and brachytherapy (a) with or without concurrent chemotherapy.
- 2: The second step depends on surgical findings.
- 2: The next step is surveillance.
For patients with cervical cancer stages IB3 and IIA2:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
- or 1cC, d: An alternative is a surgical treatment (1) with RH (cC) and pelvic lymphadenectomy (d) with or without para-aortic lymphadenectomy.
- or 1a, b: An alternative is a radiotherapy (1) with EBRT (b) and brachytherapy (a) with or without concurrent chemotherapy and adjuvant hysterectomy.
- 2: The second step depends on surgical findings.
- 2: The next step is surveillance.
Sector 3: Primary Therapy for Stages IB3, IIA2, IIB, III, and IV Based on Imaging or Surgical Staging (Figure 7)
For patients with cervical cancer stages IB3, IIA2, IIB, III, and IV and negative adenopathy:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
For patients with cervical cancer stages IB3, IIA2, IIB, III, and IV and positive adenopathy:
- 1a: Primary treatment is additional workup with imaging (1a). Further steps depend on the results.
For patients with cervical cancer stages IB3, IIA2, IIB, III, and IV and negative surgical staging:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with pelvic EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
For patients with cervical cancer stages IB3, IIA2, IIB, III, and IV and positive surgical staging:
- 1e: Primary treatment depends on LN status after surgical staging (1e).
Sector 4: Adjuvant Therapy Based on Surgical Findings (Figure 8)
For patients with cervical cancer and negative nodes, margins, and parametrium, the adjuvant therapy is:
- 1: The first step is observation if there are no risk factors (1).
- 1b: Radiotherapy (1) with EBRT (b) with or without concurrent chemotherapy if risk factors are present. According to the Sedlis criteria, those risk factors include tumor size, stromal invasion, and LVSI.
- 2: The next step is surveillance.
For patients with cervical cancer and positive pelvic nodes, margins, or parametrium, the adjuvant therapy involves:
- 1a: Primary treatment is additional workup with imaging (1a).
- 1a, b: Radiotherapy (1) with EBRT (b) and concurrent chemotherapy with or without vaginal brachytherapy (a).
- 2: The next step is surveillance.
For patients with cervical cancer and positive para-aortic nodes and negative distant metastasis, the adjuvant therapy involves:
- 1a: Primary treatment is additional workup with imaging (1a).
- 1a, b: Radiotherapy (1) with EBRT (b) and concurrent chemotherapy with or without vaginal brachytherapy (a).
For patients with cervical cancer and positive para-aortic nodes and positive distant metastasis, the adjuvant therapy involves:
- 1a: Primary treatment is additional workup with imaging (1a) and multiple biopsies (b).
- 1a, b: Radiotherapy (1) with extended-field EBRT (b) and concurrent chemotherapy with or without brachytherapy (a).
- or 1±a: An alternative is systemic therapy (1) with or without (±) radiotherapy with EBRT (a).
Sector 5: Primary Therapy Based on Imaging Results Demonstrating Positive Adenopathy (Figure 9)
For patients with cervical cancer and positive pelvic LN but negative para-aortic LN based on imaging:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with pelvic EBRT (b) and brachytherapy (a) with concurrent chemotherapy with or without para-aortic LN EBRT.
- or 1: An alternative is surgical staging (1) of para-aortic LN.
- 2: The second step is surveillance.
For patients with cervical cancer and positive pelvic LN and para-aortic LN based on imaging:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with extended-field EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
- 2: The second step is surveillance.
For patients with cervical cancer and positive adenopathy and distant metastases based on imaging:
- 1a: Primary treatment is systemic therapy (1) with radiotherapy (a).
- ±1d: With or without (±) individualized radiotherapy (1d).
Sector 6:Primary Therapy Based on Surgical Lymph Node Status (Stage IIICp) (Figure 10) For patients with stage IIIC1p cervical cancer with positive pelvic LN but negative para-aortic LN based on surgical LN status:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with pelvic EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
For patients with stage IIIC2p cervical cancer with positive para-aortic LN and negative distant metastasis based on surgical LN status:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with extended-field EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
For patients with cervical cancer with positive para-aortic LN and positive distant metastasis on imaging but not by biopsy:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with extended-field EBRT (b) and brachytherapy (a) with concurrent chemotherapy.
For patients with Stage IIIC2p cervical cancer with positive para-aortic LN and positive distant metastasis on imaging and biopsy:
- 1: Primary treatment is systemic therapy (1).
- ±1d: With or without (±) individualized radiotherapy (1d).
Sector 7: Incidental Surgical Finding of Cervical Cancer Post-Hysterectomy (Figure 11) For patients with an incidental finding of Stage IA1 cervical cancer with negative LVSI post-hysterectomy:
- 1: Management involves surveillance (1).
For patients with an incidental finding of stage IA1, IA2, or IB cervical cancer with positive LVSI but negative margins and negative imaging post-hysterectomy:
- 1b: Primary treatment is radiotherapy (1b).
- 1a, b: Radiotherapy (1) with extended-field EBRT (b) and brachytherapy (a) with or without concurrent chemotherapy.
- or 2: An alternative is a surgical treatment (2) with parametrectomy, vaginectomy, and pelvic lymphadenectomy with or without para-aortic lymphadenectomy.
- or 2b, a: Another alternative is radiotherapy (2) with pelvic EBRT (b) and brachytherapy (a) with or without concurrent chemotherapy.
- 3: The next step is observation (3) if negative LN and no residual disease.
- 3b: The next step is radiotherapy (3) with pelvic/para-aortic EBRT (b) with or without brachytherapy and concurrent chemotherapy if positive LN or residual disease.
For patients with an incidental finding of Stage IA1, IA2, or IB cervical cancer with positive LVSI, margins, and imaging post-hysterectomy:
- 1a: Primary treatment is additional workup with imaging (1a) and further management depends on the findings.
Sector 8: Stage IVB or Distant Metastases (Figure 12) For patients with stage IVB cervical cancer or distant metastases amenable to local treatment:
- 1g: Primary treatment is surgical resection (1g).
- ±1b: With or without (±) radiotherapy (1) with individualized EBRT (b).
- 1: An alternative is a surgical treatment (1) with local ablation.
- ±1b: With or without (±) radiotherapy (1) with individualized EBRT (b).
- or 1b: Or radiotherapy (1) with individualized EBRT (b) alone.
- ±1: With or without (±) systemic therapy (1).
- #2: Consider (#) adjuvant therapy (2) with systemic therapy.
- 3: The next step is surveillance (3).
For patients with stage IVB cervical cancer or distant metastases not amenable to local treatment:
- 1: Primary treatment is systemic therapy (1).
- or 1: An alternative is supportive care (1).
Sector 9: Surveillance of Persistent or Recurrent Disease (Figure 13) Surveillance of persistent or recurrent disease involves:
- 1a, e: Primary treatment is additional workup (1) with imaging (a) and surgical exploration (e).
- 1: This is followed by therapy for relapse (1).
Sector 10: Therapy for Relapse (Figure 14) For patients with local/regional recurrence of cervical cancer with no prior RT:
- #1g: Consider (#) surgical treatment (1) with resection (g).
- 1b, a: An alternative is radiotherapy (1) with individualized EBRT (b) with or without brachytherapy (a).
- ±1: With or without (±) systemic therapy (1).
For patients with central-local/regional recurrence of cervical cancer with prior RT:
- 1f, g: Primary therapy is surgical treatment (1) with pelvic exenteration (f, g).
- ±1c: With or without (±) radiotherapy (1) with IORT (c).
- ±1cC: An alternative is a surgical treatment (1) with RH (cC)
- or 1a: Or radiotherapy (1) with brachytherapy (a).
- or 2: Or supportive care.
For patients with non-central local/regional recurrence of cervical cancer and prior RT:
1b: Primary therapy is surgical treatment (1) with pelvic exenteration (f, g).
- ±1: With or without (±) systemic therapy (1).
- or 1g: An alternative is a surgical therapy (1) with resection (g).
- ±1c: With or without (±) alternative is radiotherapy (1) with IORT (c).
- or 1: Another alternative is systemic therapy (1).
- or 2: Or supportive care.
For patients with re-recurrence of cervical cancer:
- 1: Primary therapy is systemic therapy (1).
- or 1: An alternative is supportive care (1).
Discussion
A good staging system allows comparing outcomes of patients with similar characteristics within one center or across multiple centers [6]. Most guidelines including ASCO, BGCS, and NCCN use the FIGO 2018 classification for cervical cancer [1, 4, 6, 7, 9, 10, 17]. Before 2018, FIGO staged cervical cancer mainly based on clinical findings; however, in the recently updated staging system, pathological and imaging findings are taken into consideration [1,6,7]. Guidelines updated before 2018, such as ESGO and ESMO, rely on the Tumors, Nodes, and Metastases (TNM) staging due to this reason [5,13].
Patients with CC must be assessed with a thorough history and examination in addition to laboratory investigations and imaging studies [4,9,10,17,18]. Laboratory investigations include a complete blood count, liver and renal function tests [4,10,18]. Imaging includes chest x-ray, computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography/CT (PET/CT) [4,6,9,10,17-19]. MRI is superior to CT in detecting nodal disease but PET/CT is most sensitive [10,19-21]. Pre-operative PET/CT can help detect up to 54.8% of distant metastasis in CC [3].
Surgery is an option for early-stage CC. One option is conization and it aims to achieve negative margins [10,18,20,21]. Another option is hysterectomy, and there are three options: simple, modified radical, and radical [4,7]. Treating early-stage CC with RH and pelvic LND does not preserve fertility [17]. Fertility-sparing treatment is reserved for women with early-stage CC and good prognostic factors with no nodal disease [13,22]. It is not recommended for women with stage IB2 or IIA1 [10,18-20]. LVSI must be ruled out and the tumor has to measure 2 cm or less [10,18]. The presence of LVSI does not affect the staging of CC but it does affect treatment and the subsequent outcome [10]. Radical trachelectomy entails the resection of the cervix, part of the upper vagina, and the proximal parametria. It is ideally performed in the absence of LVSI, negative margins, and absent nodal disease [22]. Patients need to be counseled on the available treatment modalities and alternatives suitable for their individual case and their risks and benefits [10]. Neoadjuvant chemotherapy can help reduce the size of the tumor prior to surgery and can convert a non-fertility-sparing procedure into a more conservative approach [9,10,18,21].
Sentinel lymph node (SLN) biopsy involves removing the first-draining LNs. SLN biopsy has a high sensitivity in detecting metastasis in tumors measuring 2cm or less. It can be more reliable than LND to determine the nodal status [10]. Adjuvant therapy after surgery is indicated for patients with microscopic margins, nodal disease, and larger tumors [9,10]. It aims to reduce the risk of recurrence and increase the chance of cure [10]. Sedlis criteria can help determine those advised to receive adjuvant therapy as those with high risks including large tumor size, deep stromal invasion, and the presence of LVSI [4,5,7,10,13].
A total dose of radiotherapy administered should be 45-50 Gy [4,10,18,20,21]. Brachytherapy is a key component of RT, especially in patients with Stage IB1 or higher [2,19,20]. Treating women with advanced CC involves RT, including EBRT and BT, and platinum-based chemotherapy [10,20]. Recommended chemotherapy regimens usually include cisplatin with or without paclitaxel with or without bevacizumab [4,5,9,10,17,21]. Adding bevacizumab to the chemotherapy regimen is associated with improved overall survival, with more patients with a complete response without significantly reducing the quality of life [23].
Recurrence can either occur locally or distant and even both [7]. The most common site of recurrence is the pelvis [7,24]. The most common sites for distant metastasis include the lung, para-aortic LNs, and the abdominal cavity [8]. Treatment for recurrence depends on the primary therapy and the location of recurrence [9,10,14,18]. Unlike distant metastases, pelvic recurrences can be potentially cured with pelvic exenteration or radiation [5,8-10,19,21,24]. More than 90% of patients with distant metastasis will die within 5 years. Early-stage CC tends to metastasize locoregionally but can still metastasize to distant sites. Predictors of recurrence include tumor diameter, depth of stromal invasion, and the presence or absence of LVSI. If there is a high risk of recurrence after surgery, then post-operative adjuvant EBRT of the pelvis with or without chemotherapy with or without BT [8].
The aim of surveillance is to detect recurrent disease early when it can be effectively managed [8,13,14]. In a study by Gee et al., 13.7% of patients with locally advanced CC had “unsuspected distant metastases” [3]. The majority of recurrence occurs within 2-3 years after completing treatment and up to 95% of patients are symptomatic on presentation [10,14,18,24]. Follow-up is recommended every 3-4 months for the first two years after completing treatment and every 6-12 months for three more years and then annually or as deemed appropriate based on the patient’s individual risk of recurrence [4,5,9,10,13,14,19-21].
Managing patients with CC requires a multi-disciplinary team, which consists of a gynecologist, gynecologic oncologist, histopathologist, and radiologist, among others [10,13]. It is important to consider that not all patients have access to such services, especially in isolated settings and developing countries. We designed the guide wheel to reflect the recommendations for a highly resourced setting. This wheel is intended to facilitate the referral to established recommendations on managing patients with CC. It is not meant, in any way, to replace algorithms set by the guidelines mentioned. Although this guide wheel was designed during the COVID-19 pandemic, it does not offer any recommendations on changes in practice during this time.
- Manganaro L, Lakhman Y, Bharwani N, Gui B, Gigli S, et al. (2021) Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated guidelines of the European Society of Urogenital Radiology after revised FIGO staging. Eur Radiol 31: 7802-7816. Link: https://bit.ly/3gyRDcx
- LaVigne AW, Triedman SA, Randall TC, Trimble EL, Viswanathan AN (2017) Cervical cancer in low and middle income countries: Addressing barriers to radiotherapy delivery. Gynecol Oncol Rep 22: 16-20. Link: https://bit.ly/3uuGW2V
- Gee MS, Atri M, Bandos AI, Mannel RS, Gold MA, et al. (2018) Identification of Distant Metastatic Disease in Uterine Cervical and Endometrial Cancers with FDG PET/CT: Analysis from the ACRIN 6671/GOG 0233 Multicenter Trial. Radiology 287: 176-184. Link: https://bit.ly/3HCBf6K
- Abu-Rustum NR, Yashar CM, Bradley K, Brooks R, Campos SM, et al. (2021) Cervical Cancer, Version 1. NCCN; Pennsylvania: Link: https://bit.ly/34JrR2k
- Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Marin A, et al. (2017) Cervical cancer: ESMO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28: iv72-iv83. Link: https://bit.ly/3owTT8F
- Bhatla N, Berek JS, Fredes MC, Denny LA, Grenman S, et al. (2019) Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 145: 129-135. Link: https://bit.ly/3GtrTc0
- Bhatla, N, Aoki D, Sharma DN, Sankaranarayanan R (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143: 22-36. Link: https://bit.ly/3uyKLUw
- Elit L, Fyles AW, Devries MC, Oliver TK, Fung-Kee-Fung M, Gynecology Cancer Disease Site Group (2009) Follow-up for women after treatment for cervical cancer: a systematic review. Gynecol Oncol 114: 528-535. Link: https://bit.ly/3soOqBL
- Chuang LT, Temin S, Camacho R, Dueñas-Gonzalez A, Feldman S, et al. (2016) Management and Care of Women with Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. J Glob Oncol 2: 311-340. Link: https://bit.ly/3BjDE41
- Reed N, Balega J, Barwick T, Buckley L, Burton K, et al. (2021) British Gynaecological Cancer Society (BGCS): Cervical Cancer Guidelines: Recommendations for practice. Eur J Obstet Gynecol Reprod Biol 256: 433-465. Link: https://bit.ly/3B3y0m8
- Funston, G, O’Flynn H, Ryan NAJ, Hamilton W, Crosbie EJ (2018) Recognizing gynecological cancer in primary care: risk factors, red flags, and referrals. Adv Ther 35: 577-589. Link: https://bit.ly/3LhNs2H
- Ismail MS, Hsu S, AlKhalifa MA, Fouad M, Codabux F, et al. (2020) Evaluation of different Guidelines for cervical cancer screening and management of abnormal cervical cytology. Ann Cytol Pathol 5: 001-012. Link: https://bit.ly/3osXsMU
- Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, et al. (2018) The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Virchows Arch 472: 919-936. Link: https://bit.ly/3GtDXdr
- Salani R, Khanna N, Frimer M, Bristow RE, Chen LM (2017) An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol 146: 3-10. Link: https://bit.ly/3ovZtb8
- Ismail MS, Ismail F, Ismail J, Rustogi A, Torsten U, et al. (2020) Development of a management guideline wheel for uterine neoplasms based on different guidelines for management of uterine neoplasms. Cancer Rep Rev 4: 1-27. Link: https://bit.ly/34qvNp9
- Ismail MS (2020) Development of a management guideline wheel for abnormal pap smear and related cervical pathology. Ann Cytol Pathol 5: 013-034. Link: https://bit.ly/34GjqoC
- Johnson CA, James D, Marzan A, Armaos M (2019) Cervical cancer: An overview of pathophysiology and management. Semin Oncol Nurs 35: 166-174. Link: https://bit.ly/3Lfd1S9
- Alberta Health Services. Cancer of the uterine cervix. Alberta: AHS.
- Lim MC, Lee M, Shim SH, Nam EJ, Lee JY, et al. (2017) Practice Guidelines for Management of Cervical Cancer in Korea: A Korean Society of Gynceologic Oncology Consensus Statement. J Gyencol Oncol 28: e22. Link: https://bit.ly/3JcxF3C
- Chopra SJ, Mathew A, Maheshwari A, Bhatla N, Singh S, et al. (2018) National Cancer Grid of India Consensus Guidelines on the Management of Cervical Cancer. J Glob Oncol 4: 1-15. Link: https://bit.ly/3LtUmlV
- de Juan A, Redondo A, Rubio MJ, García Y, Cueva J, et al. (2020) SEOM clinical guidelines for cervical cancer (2019). Clin Transl Oncol 22: 270-278. Link: https://bit.ly/3gtf479
- Bentivegna E, Gouy S, Maulard A, Chargari C, Leary A, et al. (2016) Oncological outcomes after fertility-sparing surgery for cervical cancer: a systematic review. Lancet Oncol 17: e240-253. Link: https://bit.ly/3LhZUQk
- Marquina G, Manzano A, Casado A (2018) Targeted agents in cervical cancer: Beyond bevacizumab. Curr Oncol Rep 20: 40. Link: https://bit.ly/3Je4YmC
- Elit L, Reade CJ (2015) Recommendations for Follow-up Care for Gynecologic Cancer Survivors. Obstet Gynecol 126: 1207-1214. Link: https://bit.ly/3rzSNe2
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
