Annals of Cytology and Pathology
Combined HPV and CINtec PLUS testing for triaging cervical cancer screening in a Belgian cohort
Louise Cras1, Stefanie Brock1, Kurt Barbé2, Hanne Locy1, Glenn Broeckx3 and Shaira Sahebali1,4*
2Biostatistics and Medical Informatics Research Group (BISI), Vrije Universiteit Brussel (VUB), 1090 Brussels, Belgium
3Department of Pathology, Ziekenhuis Netwerk Antwerpen (ZNA), 2020 Antwerp, Belgium
4Department of Pathology, Universitair Ziekenhuis Antwerpen (UZA), 2650 Antwerp, Belgium
Cite this as
Cras L, Brock S, Barbé K, Locy H, Broeckx G, et al. (2023) Combined HPV and CINtec PLUS testing for triaging cervical cancer screening in a Belgian cohort. Ann Cytol Pathol 8(1): 004-010. DOI: 10.17352/acp.000028Copyright License
© 2023 Cras L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Cytological screening with Human Papillomavirus (HPV) triage for equivocal results has been the routine screening procedure for cervical cancer for years worldwide. The dual-marker stain p16/Ki67 (CINtec PLUS) has been shown to offer high sensitivity and specificity in the triage of women at risk of developing HPV-related precancerous lesions. We evaluated the utility of CINtec PLUS in women with normal cytology and a positive HPV test, to see if this test can be used as a prognostic biomarker.
Methods: Women of 18 years or older were assembled between January 2018 and December 2022 at two different study sites. These were cytology negative for intra-epithelial Neoplasia (NILM) and a positive HPV test. The prognostic value of the CINtec PLUS test for NILM samples and the confounding effect of HPV subtype, age, university, and follow-up stage were evaluated.
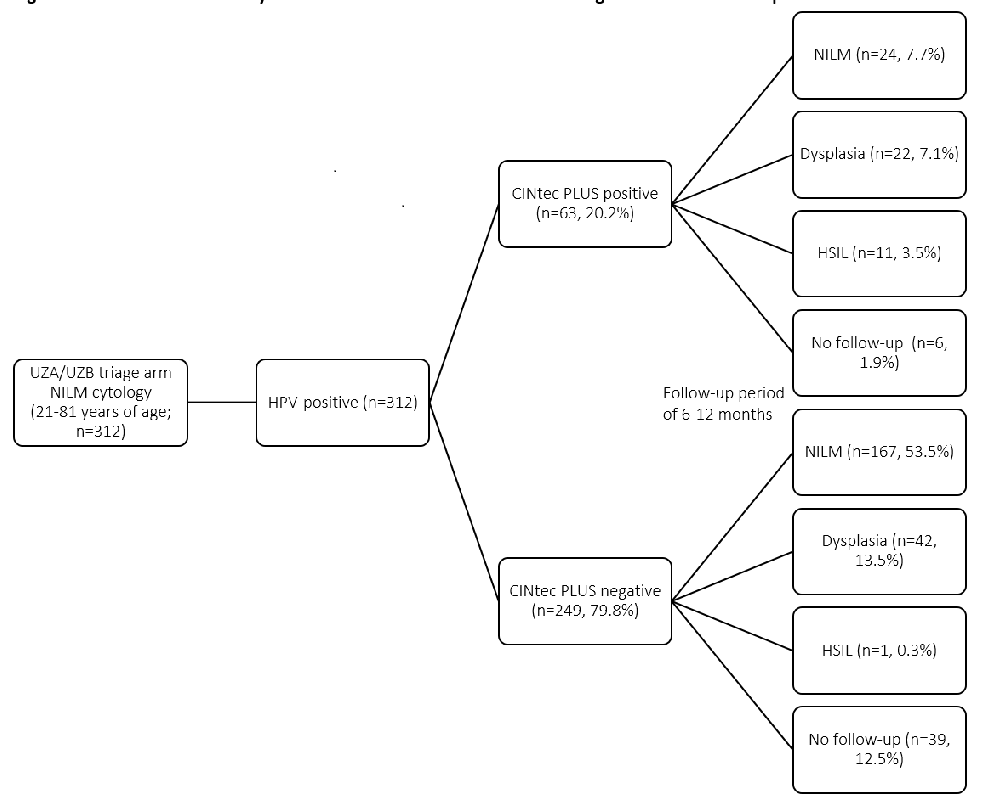
Results: CINtec PLUS was positive in 63 out of 312 (20%) NILM/ HPV-positive cases. The Positive Predictive Value (PPV) of CINtec PLUS was 92% for high-grade dysplastic lesion (HSIL), 64% for NILM, and 31% for low-grade dysplasia. The negative predictive value was 83% for HSIL, 64% for NILM, and 83% for low-grade dysplasia.
Conclusion: Adding CINtec PLUS with NILM cytology and HPV-positive test can be an important prognostic tool to identify women at risk for a high-grade dysplastic cervical lesion. Importantly, the test can also be used in primary HPV screening programs. However, women with low-grade dysplasia remain at risk for over- and under-treatment.
Abbreviations
ASC-H: Atypical Squamous Cells, cannot rule out high-grade squamous intra-epithelial lesion; ASC-US: Atypical Squamous Cells of Undetermined Significance; HPV: Human Papillomavirus; HR: High-Risk; HSIL: High-Grade Intraepithelial Lesion; NILM: Negative for Intra-Epithelial Neoplasia or Malignancy; LR-HPV: Low-Risk Human Papillomavirus; LBC: Liquid-Based Cytology; LSIL: Low-Grade Intraepithelial Lesion; PAP-test: Papanicolaou-test; SCC: Squamous Cell Carcinoma
Introduction
Cervical cancer remains a major cause of death worldwide, despite the implementation of primary and secondary prevention strategies [1]. Cervical HPV infections are common, almost all women will get infected in their lifetime [2,3]. The HPV family consists of a group of more than 200 related viruses, divided into Low-Risk (LR) HPVs and High-Risk (HR), oncogenic HPVs. These last have the potential to interfere with the normal function of the cell cycle and cause uncontrolled proliferation, leading to premalignant epithelial lesions. In particular, HPV16+ and HPV18+ strains are responsible for most HPV-related cervical cancers [3].
Normally, the immune system scans for foreign material. However, sometimes the infected cells can escape immune surveillance and evolve into a precancerous lesion [4]. Carcinogenesis is a multi-step process. It takes several years since malignant cells can be in an equilibrium stage with the immune system or can remain dormant [5]. Screening strategies for the detection of pre-malignant cervical neoplasia as well as the use of prophylactic vaccines against several HR-HPV subtypes have proven successful to prevent lesions [6]. Several techniques are being used in population-wide screening programs to reduce morbidity and mortality from cervical cancer, the most important being cytology and HPV testing [7].
Increasing numbers of cytology negative for intraepithelial Neoplasia (NILM) are complemented by HPV testing on demand of the patient or clinician in order to identify more at-risk cases. This leads to an increased number of HPV-positive cases, with a subsequent referral for colposcopy. These women are at risk for overtreatment in the absence of dysplasia, indicating the importance of refining the triage and management of these women [8,9]. Therefore, it is important to elucidate, why, when, and how an HPV infection becomes oncogenic.
Ijkenberg and McMenamin, et al. investigated the use of CINtec PLUS on NILM cytology samples [6,10]. The CINtec PLUS test uses two markers, p16, and Ki67, and has been shown to have increased total sensitivity (66% in Belgium) compared to cytology and HPV testing in detecting cervical dysplasia [9,10]. In normal cells, p16 has an antiproliferative effect and arrests the cell cycle after mitosis. In cells infected by HR-HPV which has become oncogenic, cell cycle arrest does not occur. This inhibits a negative feedback control mechanism, resulting in over-expression of p16. Ki67 is a proliferation marker that can be detected in the nuclei of dividing cells and gives insight into the activation of the cell cycle [11,12]. Simultaneous detection of these biomarkers is indicative of an aberration in the cell cycle and oncogenic, HPV-related transformation [13].
In the summer of 2022, the federal Minister of Health announced plans for a new screening program, based on HR-HPV testing, so-called HPV primary screening. In such a program, the triaging problem of HPV-positive NILM samples will still be present and may even become more poignant. This study aims to investigate the prognostic value of the CINtec PLUS test in women with NILM cytology and HPV-positivity and its possible clinical implementation in the current screening programme.
Materials and methods
The study was approved by the Ethics Committee of the University Hospital Brussels (B.U.N. 1432022000100 / EC number: EC-2022-134.
Study population
Women were recruited via the Departments of Pathology at the University Hospital of Antwerp (UZ Antwerp) and the University Hospital of Brussels (UZ Brussel). 152 cases from cervical screening (Brussels) and 160 cases from both cervical screening and follow-up cervical cytology (Antwerp) were included in the cohort. Both populations included women aged 18 years and above. Retrospectively, consecutive series of cytology samples that were diagnosed as NILM (negative for intraepithelial lesion or malignancy) with a positive additional HPV test for high-risk HPV subtypes, requested by either the clinician or the patients, were included in the cohort. Data from follow up cytology and/or biopsy were collected. The follow-up period was 6 months to 1 year after the initial screening result.
In the UZA, on clinical request, a CINtec PLUS test was performed on HPV-positive cases following the manufacturer’s protocol (see below). HPV-positive cases with NILM cytology from UZ Brussels were sent for CINtec PLUS cytology analysis at the pathology lab in UZ Antwerp. In the UZ Brussels series, the CINtec data were not used in a clinical setting.
All samples were pseudo-anonymized prior to enrollment in the study.
Women with a history of previous gynecologic tumour, gynecologic radiotherapy, or any kind of therapy in the pelvic region, were not included in the cohort. All samples with abnormal/ borderline cytology and non-representative samples were excluded.
Cytology
In both labs, cytology testing was performed using the ThinPrep technique with the T5000 Processor (Hologic®). This system is used to process liquid-based cytology samples into monolayer slides. After completing the ThinPrep technique a PAP stain (polychromatic cytological stain) is performed on the Sakura® stainer. Cytology samples were analysed by a qualified and trained cytologist and cytopathologist and interpreted according to the Bethesda System.
HPV testing
In UZB, HPV testing was performed using the Cobas 4800 HPV test (Roche®) which is a CE-IVD labeled, qualitative in vitro test for the detection of HPV in patient samples. The assay uses the amplification of target DNA by PCR and nucleic acid hybridisation to detect 14 HR HPV types in one assay. This test specifically identifies HPV 16 and HPV 18, while simultaneously detecting ‘other high-risk HPV subtypes (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) at clinically relevant levels of infection.
In UZA testing for HR-HPV was performed with the Cepheid HPV Xpert test. This is a comparable technique to the Cobas test, but it tests separately for the 14 different HR-HPV types. For this study, results were grouped like the Cobas results, to enable comparisons.
CINtec PLUS immunocytochemistry
CINtec PLUS (Roche®) combines two biomarkers: a mouse monoclonal antibody p16 (p16INK4a) and a proliferation biomarker, a rabbit monoclonal antibody Ki67. The kit includes a DAB brown detection chemistry for p16 and a fast red detection chemistry for Ki67. The brown color for p16 will stain in the cytoplasm and nucleus or only in the cytoplasm and the red color for Ki67 will stain only in the nucleus [10]. If simultaneously expressed, the markers indicate the effect of carcinogenically transforming HPV infections [3]. The test was performed on the Ventana BenchMark ULTRA IHC/ISH system (Roche®).
The CINtec PLUS test was considered positive when at least one dual-stained cervical cell was present (brown cytoplasmic staining for p16 and red nuclear staining for Ki67). The test was considered negative when staining was completely absent or if only one biomarker was present (brown cytoplasmic staining for p16 or red nuclear staining for Ki67) [6,10].
Dual-stained cytology was performed on residual cellular material out of the LBC vial collected at the initial screening visit [14]. Interpretation of the dual stain marker was highly reproducible with minimal training [15]. Samples were analysed by two trained pathologists.
Statistics and analysis
A Linear Discriminant Analysis (LDA) was used for statistical analysis performed by the software programme SPSS v. 27. For this purpose, the outcome of follow-up smears was divided into 4 variables (NILM, dysplasia, HSIL, and no-follow-up). Age, hospital (UZA or UZB), and HPV-subtype were considered confounders whereas CINtec PLUS was considered as the test to predict the outcome variable. Positive Predictive Value (PPV), negative predictive value (NPV), false positives, and false negatives were calculated. For each of the 4 different outcome variables, a separate LDA was performed.
Discussion
Overview of cohort and population characteristics
The average age for the UZ Antwerp cohort is 49,4 years (range 24-82y) and for UZ Brussels 43 years (range 21-70y). Both cohorts had similar screening and HPV test results. HPV16+ was found in 62 cases (19.9%) and had a follow-up in 56 cases, 3 of which yielded high-grade dysplasia. HPV18+ was present in 17 cases (5.4%). 14 of those had a follow-up, only 1 of which contained high-grade dysplasia. 255 cases contained HPVother+ (81.7%). Of the 214 which received follow-up, 10 were high-grade lesions. In total 191 (61.2%) of all follow-up results were NILM. 12 (3,8%) of all follow-up results were high grade (Table 1).
The CINtec PLUS test was positive in 63 women and negative in the remaining 249 women. There were no significant differences between both cohorts in CINtec results. There were no non-conclusive results, all tests were analysable and interpretable by pathologists. In the CINtec positive group, follow-up smears were NILM in 38.1%, 34.9% contained a low-grade lesion, and 17.5% an HSIL. In 9.5% there was no follow-up. In the CINtec negative group, follow-up smears were NILM in 67%, 16.9% contained a low-grade lesion and 1 case in 249 was an HSIL. 15.7% or 39 cases had no follow-up (Figures 1,2 Table 1). In all, of 12 high-grade lesions found, only 1 was CINtec negative. This case contained HPVother+.
Follow-up cytology stage and HPV subtype did not show a correlation
The relationship between the CINtec PLUS test and HPV subtype variables is represented in Table 1. Within the CINtec-positive samples with HSIL, HPV16+ was present in 4.8% of the cases, HPV18+ in 1.6% and HPVother+ in 14.3%. The low-grade dysplastic lesions showed HPV16+ in 7.9%, HPV18+ in 1.6%, and HPVother+ in 25.4%. Finally, the HPV subtype distribution in the NILM smears was HPV16+ 9.5%, HPV18+ 4.8%, and HPVother+ 30.2%. In contrast, CINtec PLUS negative samples with an HSIL follow-up smear were not present for both HPV16+ and HPV18+ and only in one case positive for HPVother subtype (0.4%). The low-grade dysplastic lesions showed HPV16+ 3.6%, HPV18+ 0.4%, and for HPVother+ 14.1%. Finally, 13.3% had HPV16+ NILM smears, HPV18+ 3.2% and HPVother+ 53.8% (Table 1). These findings were not statistically significant.
Combination of HPV and CINtec PLUS testing proves valuable to detect lesions evolving into HSIL
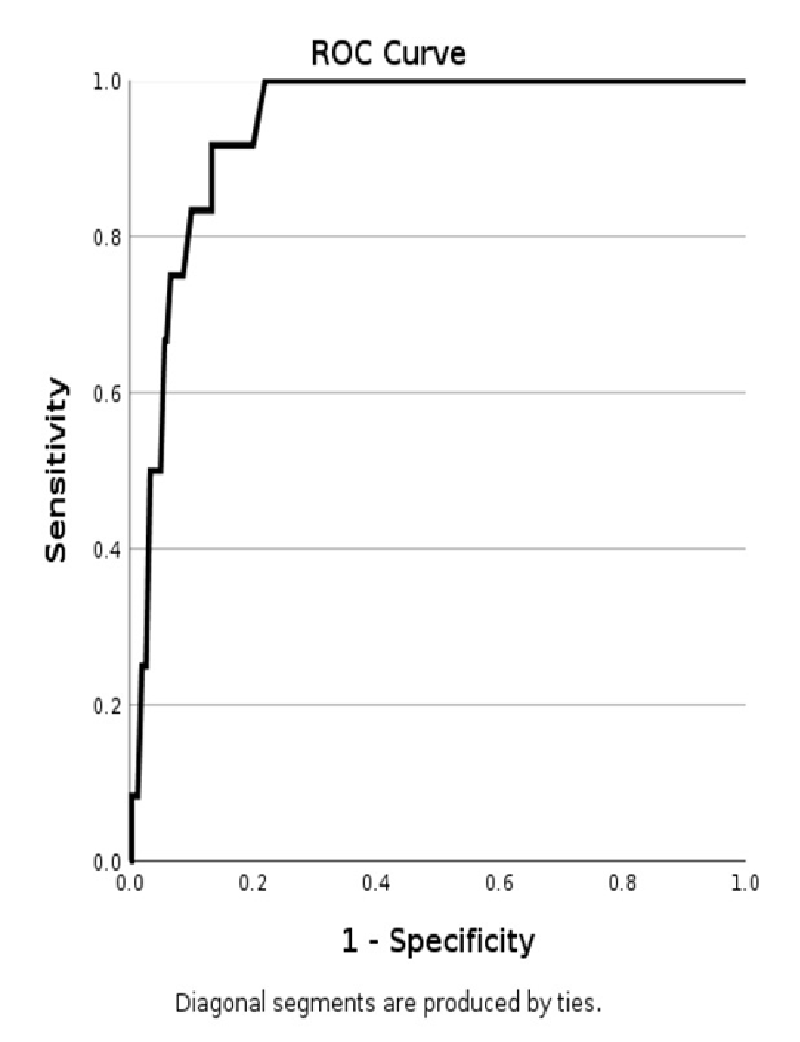
The clinical performance of CINtec PLUS triage shows a high Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of 92% and 83% for HSIL respectively, regardless of age or HPV subtypes. CINtec PLUS test resulted in an accuracy of 84% for the HSIL. This is illustrated by the receiver operating characteristic (ROC) curve (Figure 3), where sensitivity is plotted against 1-specificity. The obtained area under the curve (AUC) provides the value for the accuracy; in this case for HSIL. The low-grade cervical lesion category showed a low PPV and high NPV value of 31% and 83%, respectively, resulting in an accuracy of 73%. The follow-up smears with NILM showed a PPV and NPV value of both 64% (Table 2, Figure 3).
CINtec PLUS positivity correlated significantly (p - value of <0.001) with a follow-up cytology result of HSIL, while negative CINtec PLUS negativity was significantly related to a NILM follow-up cytology (p < 0.001). In contrast, low-grade lesions and non-follow-up lesions could not be discriminated against based on CINtec PLUS positivity or negativity. Regarding the HPV subtypes, no significant differences between HPV 16 / 18 / other were detected.
Findings
This retrospective inter-university study aimed to evaluate the clinical utility and prognostic value of CINtec PLUS immunocytochemistry in a primary screening population of 312 HPV-positive women with NILM cytology.
Current primary screening in Belgium uses cervical cytology. The sensitivity of this technique is relatively low, as it is a morphology-based technique, and rather labor-intensive even when automated [16]. Nevertheless, a cervical smear is very specific for the detection of HSIL [14]. Additional HPV testing in triage is known to significantly increase the sensitivity for the detection of HSIL but has low specificity [10,14,16]. This is especially important in young women under 30 years of age with a relatively high prevalence of often subclinical HPV infections, creating a risk for over-treatment with colposcopy [6]. In our study, there were only 45 cases (14%) without follow-up, even though they had no cytological lesion. 191 (72%) of all follow-up results were NILM. This illustrates the risk of over-treatment.
In previous studies, the sensitivity of dual-stained immunocytochemistry reached more than 90% of the sensitivity level of HPV testing. At the same time, specificity is significantly higher as compared to HPV testing, reducing the clinical false-positive HPV results and colposcopy referrals by almost 50% [10]. This is confirmed in this Flemish study, where 11 out of 12 HSILs in follow-up had a positive CINtec PLUS result. This could not have been predicted just by cytology and HPV screening results.
Previous studies showed that CINtec PLUS positivity is associated with HSIL or invasive carcinoma and that simultaneous detection of the tumour suppressor protein p16 and the proliferation marker Ki67 is associated with oncogenic HPV-associated transformation in the cervical cells [17]. In September 2020, the FDA approved the expanded use of CINtec PLUS as a triage of HPV-positive women, to clarify further clinical management. Our study in NILM samples, confirms this observation as CINtec PLUS shows high PPV (92%) and high NPV (83%) for high-grade dysplasia (HSIL). These results are promising to improve clinical follow-up for HPV-positive women with positive CINtec PLUS results.
In addition, for follow-up cytology resulting in NILM, the CINtec PLUS test shows a PPV of 64% and an NPV of 64%. These results confirm previous findings in the literature with a PPV of 66% [6]. In concordance with previous results, our data suggest that a CINtec PLUS test is a valuable tool to avoid overtreatment in women with NILM and recommend a normal screening interval to them while in women possibly harbouring a high-grade lesion, the CINtec PLUS is a valuable tool to avoid undertreatment and identify these lesions faster [6,18].
However, low-grade lesions are more difficult to differentiate by CINtec PLUS. Results in borderline or low-grade lesions (ASC-US, LSIL) show low PPV (31%) and high NPV (83%). This is understandable in light of oncogenic transformation. Only a subset of these lesions is really dysplastic. Because of this, women with such lesions are at risk for overtreatment [6,19]. There is a reduced sensitivity for low-grade lesions, in particular for women < 30 years. This needs to be more carefully considered in individual risk assessments of women. Much more research is needed in this area. However, CINtec PLUS was found to be more specific than HPV for such lesions and reduced the number of colposcopic referrals for women with LSIL [20,21].
A recent study has shown that hypermethylation of CpG islands in proximity to the human gene methylation panel (specific for CIN3 and cervical cancer) (DLX1, ITGA4, RXFP3, SOX17, and ZNF671) correlated with the presence of both low-grade and high-grade cervical lesions [22]. This gynTect study shows an excellent specificity for SCC, CIN3, and even for CIN1/2 and found a similar PPV (74,5%) value as for CINtec PLUS (69,2%) although they highlight that it did not allow differentiation between lesions prone to progression and lesions that might persist or regress. Although this can be an interesting alternative for the future, this molecular test has its boundaries as it requires more financial resources, trained lab staff for technical performance, and proper analysis. In comparison, CINtec PLUS is a relatively low-cost test that is easily implemented and can be interpreted in the same lab as the cytologic evaluation [7].
Our findings regarding the predictive value and accuracy of CINtec PLUS in HPV-positive NILM women are in correlation with previous studies that have investigated these features in different populations [23-26]. A limitation of our study is a short follow-up period of 48 months, registered from the beginning of 2018 to the end of 2022, while the development of a dysplastic lesion can be highly variable and can often take more time. One study noted that CIN2/CIN3 lesions had a short-term progression rate of +/- 30% [7]. This implies that women who currently have NILM cytology and positive CINtec can still develop HSIL after the reported follow-up period. For these women, an extended follow-up period with CINtec PLUS testing may be required, something we could not determine at this point. On the other hand, another study found that CIN2 lesions showed an overall regression rate of 50% [27].
In our study, the outcome of follow-up smears was divided into 4 groups (NILM, dysplasia, HSIL, and no follow-up). The disadvantage of working with an all-encompassing term dysplasia for LSIL, ASC-US, and ASC-H is that samples with overt reactive changes that were interpreted as ASC-US could end up under the heading of dysplasia. In this way, a bias would be created that could affect PPV, NPV and accuracy.
In contrast with large cohort studies, our study did not observe any significant differences between outcome and HPV subtype and age which can be due to our small population. Multiple other studies with separate HPV16 and HPV18 testing noted a correlation with the probability of developing an HSIL, especially in older women [28,29]. Moreover, HPV16-positive women show a higher absolute risk for cervical pre-cancer in contrast with other oncogenic HPV types, which may have clinical implications [6,30,31]. Our study population contains quite a high number of non-16/18 hrHPV types. The reason for this is unclear. There is a group of African as well as Eastern European women included, which may have influenced the type distribution. Since the study was not designed to evaluate such differences, we do not know if this could be an explanation. It could also be a legitimate shift in the HPV distribution, as part of the population is already vaccinated for HPV16/18. A third possibility is, that the HPV tests used in this study picked up more non-16/18 types, co-infections, etc. Variations in specificity for the respective types could explain this. However, we used standardised, clinically approved tests, which are applied in daily practice [32,33]. Therefore we don’t expect these tests to deviate much from expected performance.
Glandular lesions were not taken into consideration as our cohort did not include any glandular lesions in the follow-up. The value of CINtec PLUS in women with glandular lesions was evaluated elsewhere and demonstrated good prognostic results [34-37].
Notable in this study is the number of women with no follow-up smear (n = 46, 14.8%). This may be due to the initial negative smear which causes women in Belgium to be followed in a 3-year follow-up schedule. Currently, HPV infections without a concurrent dysplastic lesion are not covered by the screening guidelines. Therefore, it is unclear what management such cases need. On the other hand, it is important to properly inform the female population to reduce barriers to screening and improve screening compliance [38-40].
At the moment immunocytochemistry tests such as CINtec PLUS are not reimbursed by the RIZIV/INAMI for cervical screening purposes. This may be a reason why there is only a little information on the use of this test in the Belgian/ Flemish screening programme. If the accuracy of CINtec PLUS to identify high-grade dysplasia could be confirmed in this population, this could lead to more efficient (faster) and cost-effective screening. Less repeat testing and fewer colposcopies would be needed to confirm the presence of dysplasia.
In the nearby future, Belgium may follow other countries and introduce a cervical screening programme with HPV testing as the primary screening modality [41-43]. In such a programme triage of HPV-positive cases will still be necessary, to avoid an excess of colposcopy follow-up. This could be done with either cytology, CINtec, or a combination of tests to provide a strategy to identify those women who need follow-up.
Conclusion
This study investigated the added value of a CINtec PLUS test in 312 women with a NILM and HPV positivity. We note a significant added value for women who are at risk of developing HSIL. However, the risk of over- and undertreatment of women with low-grade dysplasia remains real. We have shown that CINtec PLUS is not only a valuable tool for equivocal screening results but also has added value for initial NILM cytology. The same holds true in a setting with HPV primary screening.
Statements
Statement of ethics: Written informed consent was not required and the retrospective analysis was undertaken after approval from the Institutional Ethics Committee on human research UZ Brussel/VUB (BUN: 1432022000100/EC number 2022-134).
Funding sources: This study was supported by a research grant from Roche providing CINtec PLUS test kits for the study. The study was investigator-initiated and conducted independently.
Author contributions: SS: Conceptualisation, Funding acquisition, Supervision, Writing-review, and editing. LC: Project administration, Investigation, Methodology, Resources, Data Analysis, Writing manuscript, and editing. SB: Data interpretation. HL: Data interpretation. KB: Data analysis and Writing-review. GB: Data collection and Writing-review.
Data availability statement: The data generated or analysed during this study are not included in this article. Further inquiries can be directed to the corresponding author.
- Zhang X, Zeng Q, Cai W, Ruan W. Trends of cervical cancer at global, regional, and national level: data from the Global Burden of Disease study 2019. BMC Public Health. 2021 May 12;21(1):894. doi: 10.1186/s12889-021-10907-5. PMID: 33975583; PMCID: PMC8114503.
- Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017 Apr;40(2):80-85. Epub 2017 Apr 3. PMID: 28368072.
- Ramakrishnan S, Partricia S, Mathan G. Overview of high-risk HPV's 16 and 18 infected cervical cancer: pathogenesis to prevention. Biomed Pharmacother. 2015 Mar;70:103-10. doi: 10.1016/j.biopha.2014.12.041. Epub 2015 Jan 12. PMID: 25776487.
- Guo L, Hua K. Cervical Cancer: Emerging Immune Landscape and Treatment. Onco Targets Ther. 2020 Aug 12;13:8037-8047. doi: 10.2147/OTT.S264312. PMID: 32884290; PMCID: PMC7434518.
- Szalmás A. Commentary: Induction of Dormancy in Hypoxic Human Papillomavirus-Positive Cancer Cells. Front Oncol. 2018 [cited 2023 Feb 7; 8. https://www.frontiersin.org/articles/10.3389/fonc.2018.00077
- McMenamin M, McKenna M, McDowell A. Clinical Utility of CINtec PLUS Triage in Equivocal Cervical Cytology and Human Papillomavirus Primary Screening. Am J Clin Pathol. 2018 Oct 24;150(6):512-521. doi: 10.1093/ajcp/aqy073. PMID: 30169728.
- Schmitz M, Eichelkraut K, Schmidt D, Zeiser I, Hilal Z, Tettenborn Z, Hansel A, Ikenberg H. Performance of a DNA methylation marker panel using liquid-based cervical scrapes to detect cervical cancer and its precancerous stages. BMC Cancer. 2018 Dec 3;18(1):1197. doi: 10.1186/s12885-018-5125-8. PMID: 30509219; PMCID: PMC6276155.
- WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. [cited 2023 Feb 7]. https://www.who.int/publications-detail-redirect/9789240030824
- Tjalma WAA. Diagnostic performance of dual-staining cytology for cervical cancer screening: A systematic literature review. Eur J Obstet Gynecol Reprod Biol. 2017 Mar;210:275-280. doi: 10.1016/j.ejogrb.2017.01.009. Epub 2017 Jan 8. PMID: 28086168.
- Ikenberg H, Bergeron C, Schmidt D, Griesser H, Alameda F, Angeloni C, Bogers J, Dachez R, Denton K, Hariri J, Keller T, von Knebel Doeberitz M, Neumann HH, Puig-Tintore LM, Sideri M, Rehm S, Ridder R; PALMS Study Group. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013 Oct 16;105(20):1550-7. doi: 10.1093/jnci/djt235. Epub 2013 Oct 4. PMID: 24096620; PMCID: PMC3814411.
- Shi Q, Xu L, Yang R, Meng Y, Qiu L. Ki-67 and P16 proteins in cervical cancer and precancerous lesions of young women and the diagnostic value for cervical cancer and precancerous lesions. Oncol Lett. 2019 Aug;18(2):1351-1355. doi: 10.3892/ol.2019.10430. Epub 2019 Jun 3. PMID: 31423197; PMCID: PMC6607340.
- Zhang R, Ge X, You K, Guo Y, Guo H, Wang Y, Geng L. p16/Ki67 dual staining improves the detection specificity of high-grade cervical lesions. J Obstet Gynaecol Res. 2018 Nov;44(11):2077-2084. doi: 10.1111/jog.13760. Epub 2018 Aug 9. PMID: 30094887.
- Wentzensen N, von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. 2007;23(4):315-30. doi: 10.1155/2007/678793. PMID: 17627065; PMCID: PMC3851733.
- Petry KU, Schmidt D, Scherbring S, Luyten A, Reinecke-Lüthge A, Bergeron C, Kommoss F, Löning T, Ordi J, Regauer S, Ridder R. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecol Oncol. 2011 Jun 1;121(3):505-9. doi: 10.1016/j.ygyno.2011.02.033. Epub 2011 Mar 21. PMID: 21420158.
- Wentzensen N, Fetterman B, Tokugawa D, Schiffman M, Castle PE, Wood SN, Stiemerling E, Poitras N, Lorey T, Kinney W. Interobserver reproducibility and accuracy of p16/Ki-67 dual-stain cytology in cervical cancer screening. Cancer Cytopathol. 2014 Dec;122(12):914-20. doi: 10.1002/cncy.21473. Epub 2014 Aug 12. PMID: 25132656.
- Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, Schünemann H, Paraskevaidis E, Arbyn M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017 Aug 10;8(8):CD008587. doi: 10.1002/14651858.CD008587.pub2. PMID: 28796882; PMCID: PMC6483676.
- Ordi J, Sagasta A, Munmany M, Rodríguez-Carunchio L, Torné A, del Pino M. Usefulness of p16/Ki67 immunostaining in the triage of women referred to colposcopy. Cancer Cytopathol. 2014 Mar;122(3):227-35. doi: 10.1002/cncy.21366. PMID: 24757722.
- McMenamin M, McKenna M, McDowell A, Dawson C, McKenna R. Intra- and inter-observer reproducibility of CINtec® PLUS in ThinPrep® cytology preparations. Cytopathology. 2017 Aug;28(4):284-290. doi: 10.1111/cyt.12426. Epub 2017 Jul 6. PMID: 28685883.
- Wentzensen N, Schwartz L, Zuna RE, Smith K, Mathews C, Gold MA, Allen RA, Zhang R, Dunn ST, Walker JL, Schiffman M. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res. 2012 Aug 1;18(15):4154-62. doi: 10.1158/1078-0432.CCR-12-0270. Epub 2012 Jun 6. PMID: 22675168; PMCID: PMC4237612.
- Gilbert L, Ratnam S, Jang D, Alaghehbandan R, Schell M, Needle R, Ecobichon-Morris A, Wadhawan A, Costescu D, Elit L, Wang P, Zahariadis G, Chernesky M. Comparison of CINtec PLUS cytology and cobas HPV test for triaging Canadian patients with LSIL cytology referred to colposcopy: A two-year prospective study. Cancer Biomark. 2022;34(3):347-358. doi: 10.3233/CBM-210366. PMID: 35001877; PMCID: PMC9535599.
- Ratnam S, Jang D, Gilbert L, Alaghehbandan R, Schell M, Needle R, Ecobichon-Morris A, Wang PP, Rahman M, Costescu D, Elit L, Zahariadis G, Chernesky M. CINtec PLUS and cobas HPV testing for triaging Canadian women referred to colposcopy with a history of low-grade squamous intraepithelial lesion: Baseline findings. Papillomavirus Res. 2020 Dec;10:100206. doi: 10.1016/j.pvr.2020.100206. Epub 2020 Aug 20. Erratum in: Tumour Virus Res. 2021 Jun;11:200215. PMID: 32828968; PMCID: PMC7548977.
- Hansel A, Steinbach D, Greinke C, Schmitz M, Eiselt J, Scheungraber C, Gajda M, Hoyer H, Runnebaum IB, Dürst M. A promising DNA methylation signature for the triage of high-risk human papillomavirus DNA-positive women. PLoS One. 2014 Mar 19;9(3):e91905. doi: 10.1371/journal.pone.0091905. PMID: 24647315; PMCID: PMC3960142.
- Loghavi S, Walts AE, Bose S. CINtec® PLUS dual immunostain: a triage tool for cervical pap smears with atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesion. Diagn Cytopathol. 2013 Jul;41(7):582-7. doi: 10.1002/dc.22900. Epub 2012 Jul 26. PMID: 22833355.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol. 2011 Jun 25;119(3):158-66. doi: 10.1002/cncy.20140. Epub 2011 Mar 25. PMID: 21442767.
- Wentzensen N, Fetterman B, Castle PE, Schiffman M, Wood SN, Stiemerling E, Tokugawa D, Bodelon C, Poitras N, Lorey T, Kinney W. p16/Ki-67 Dual Stain Cytology for Detection of Cervical Precancer in HPV-Positive Women. J Natl Cancer Inst. 2015 Sep 15;107(12):djv257. doi: 10.1093/jnci/djv257. PMID: 26376685; PMCID: PMC4675094.
- Waldstrøm M, Christensen RK, Ørnskov D. Evaluation of p16(INK4a)/Ki-67 dual stain in comparison with an mRNA human papillomavirus test on liquid-based cytology samples with low-grade squamous intraepithelial lesion. Cancer Cytopathol. 2013 Mar;121(3):136-45. doi: 10.1002/cncy.21233. Epub 2012 Sep 17. PMID: 22987560.
- Tainio K, Athanasiou A, Tikkinen KAO, Aaltonen R, Cárdenas J, Hernándes, Glazer-Livson S, Jakobsson M, Joronen K, Kiviharju M, Louvanto K, Oksjoki S, Tähtinen R, Virtanen S, Nieminen P, Kyrgiou M, Kalliala I. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ. 2018 Feb 27;360:k499. doi: 10.1136/bmj.k499. PMID: 29487049; PMCID: PMC5826010.
- Agorastos T, Chatzistamatiou K, Katsamagkas T, Koliopoulos G, Daponte A, Constantinidis T, Constantinidis TC; HERMES study group. Primary screening for cervical cancer based on high-risk human papillomavirus (HPV) detection and HPV 16 and HPV 18 genotyping, in comparison to cytology. PLoS One. 2015 Mar 20;10(3):e0119755. doi: 10.1371/journal.pone.0119755. PMID: 25793281; PMCID: PMC4368762.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011 Sep;12(9):880-90. doi: 10.1016/S1470-2045(11)70188-7. Epub 2011 Aug 22. PMID: 21865084.
- Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005 Jul 20;97(14):1066-71. doi: 10.1093/jnci/dji186. PMID: 16030304.
- Stoler MH, Wright TC Jr, Sharma A, Zhang G, Apple R, Wright TL, Behrens CM; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. The interplay of age stratification and HPV testing on the predictive value of ASC-US cytology. Results from the ATHENA HPV study. Am J Clin Pathol. 2012 Feb;137(2):295-303. doi: 10.1309/AJCPGW1V2BBWMOCX. PMID: 22261457.
- Rabaan AA, Alfaraj SA, Alkhalifah MA. Comparison of the Cepheid Xpert HPV test and the HC2 High-Risk HPV DNA Test for detection of high-risk HPV infection in cervical smear samples in SurePath preservative fluid. J Med Microbiol. 2018 May;67(5):676-680. doi: 10.1099/jmm.0.000723. PMID: 29580367; PMCID: PMC5994697.
- Arbyn M, Snijders PJ, Meijer CJ, Berkhof J, Cuschieri K, Kocjan BJ, Poljak M. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect. 2015 Sep;21(9):817-26. doi: 10.1016/j.cmi.2015.04.015. Epub 2015 May 1. PMID: 25936581.
- Aromseree S, Wongjumpa W, Ekalaksananan T, Temtanakitpaisan A, Kleebkaow P, Srisathaporn S, Tongchai P, Pientong C. P16/Ki-67 Dual Staining in Positive Human Papillomavirus DNA Testing for Predictive Diagnosis of Abnormal Cervical Lesions in Northeastern Thai Women. Asian Pac J Cancer Prev. 2022 Oct 1;23(10):3405-3411. doi: 10.31557/APJCP.2022.23.10.3405. PMID: 36308365; PMCID: PMC9924320.
- Singh M, Mockler D, Akalin A, Burke S, Shroyer A, Shroyer KR. Immunocytochemical colocalization of P16(INK4a) and Ki-67 predicts CIN2/3 and AIS/adenocarcinoma. Cancer Cytopathol. 2012 Feb 25;120(1):26-34. doi: 10.1002/cncy.20188. PMID: 22162342.
- Ravarino A, Nemolato S, Macciocu E, Fraschini M, Senes G, Faa G, Negri G. CINtec PLUS immunocytochemistry as a tool for the cytologic diagnosis of glandular lesions of the cervix uteri. Am J Clin Pathol. 2012 Nov;138(5):652-6. doi: 10.1309/AJCP00INMGIFYFNQ. PMID: 23086765.
- Ryu A, Honma K, Shingetsu A, Tanada S, Yamamoto T, Nagata S, Kamiura S, Yamasaki T, Ohue M, Matsuura N. Utility of p16/Ki67 double immunocytochemistry for detection of cervical adenocarcinoma. Cancer Cytopathol. 2022 Dec;130(12):983-992. doi: 10.1002/cncy.22631. Epub 2022 Aug 17. PMID: 35976043.
- Akinlotan M, Bolin JN, Helduser J, Ojinnaka C, Lichorad A, McClellan D. Cervical Cancer Screening Barriers and Risk Factor Knowledge Among Uninsured Women. J Community Health. 2017 Aug;42(4):770-778. doi: 10.1007/s10900-017-0316-9. PMID: 28155005; PMCID: PMC5494033.
- Uijterwaal MH, Polman NJ, Witte BI, van Kemenade FJ, Rijkaart D, Berkhof J, Balfoort-van der Meij GA, Ridder R, Snijders PJ, Meijer CJ. Triaging HPV-positive women with normal cytology by p16/Ki-67 dual-stained cytology testing: baseline and longitudinal data. Int J Cancer. 2015 May 15;136(10):2361-8. doi: 10.1002/ijc.29290. Epub 2014 Nov 6. PMID: 25345358.
- Petry KU, Barth C, Wasem J, Neumann A. A model to evaluate the costs and clinical effectiveness of human papilloma virus screening compared with annual papanicolaou cytology in Germany. Eur J Obstet Gynecol Reprod Biol. 2017 May;212:132-139. doi: 10.1016/j.ejogrb.2017.03.029. Epub 2017 Mar 19. PMID: 28363186.
- Maver PJ, Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect. 2020 May;26(5):579-583. doi: 10.1016/j.cmi.2019.09.006. Epub 2019 Sep 17. PMID: 31539637.
- Ntanasis-Stathopoulos I, Kyriazoglou A, Liontos M, A Dimopoulos M, Gavriatopoulou M. Current trends in the management and prevention of human papillomavirus (HPV) infection. J BUON. 2020 May-Jun;25(3):1281-1285. PMID: 32862567.
- Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, Ceballos K, Quinlan D, Lee M, Martin RE, Gentile L, Peacock S, Stuart GCE, Franco EL, Coldman AJ. Effect of Screening With Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial. JAMA. 2018 Jul 3;320(1):43-52. doi: 10.1001/jama.2018.7464. Erratum in: JAMA. 2018 Dec 4;320(21):2273. PMID: 29971397; PMCID: PMC6583046.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
