Annals of Cytology and Pathology
The association between tumor microenvironment collagen and liver metastasis in colorectal cancer
Jiawen Fan, Qing Zhu, Jianming Nie and J Dinghua Yang*
Cite this as
Fan J, Zhu Q, Nie J, Yang JD (2024) The association between tumor microenvironment collagen and liver metastasis in colorectal cancer. Ann Cytol Pathol 9(1): 013-019. DOI: 10.17352/acp.000031Copyright License
© 2024 Fan J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The colon is an organ rich in collagen, while the liver is an organ deficient in collagen. The tissue microenvironment of the two organs differs significantly, but the incidence of liver metastasis in colorectal cancer is high. Besides vascular drainage factors, the changes in collagen occurring in the liver during the process of colorectal cancer liver metastasis are also very important. This article aims to discuss the characteristics of collagen changes in the premetastatic stage, liver colonization, and intrahepatic metastasis process of colorectal cancer liver metastasis.
Introduction
Colorectal Cancer (CRC) is the third most common cancer worldwide [1]. The liver is the primary target organ for the metastasis of colorectal cancer, and liver metastasis is the main cause of death in colorectal cancer patients. Approximately 40-50% of colorectal cancer patients experience liver metastasis during the disease [2]. Even after undergoing R0 tumor resection surgery, more than half of the patients still experience recurrence within 2 years, with a high recurrence rate of 50-80% [3-5]. With the wide acceptance of Parenchymal-Sparing Hepatectomy (PSH) [6-8] and the concept of multiple resections after liver metastasis recurrence, the surgical decision for treating CLMR largely depends on disease prediction. Therefore, there is a need for more detailed, personalized, and accurate prognosis prediction for patients, which has direct clinical significance.
Extracellular Matrix (ECM) as the most abundant component in the tumor microenvironment can regulate tumor cell behavior and tissue tension homeostasis. Collagen is the main functional component of ECM, forming the scaffold for various cell growth and the bridge for cell movement in the tumor microenvironment. Through collagen degradation and redeposition, ECM remodeling is regulated, promoting tumor infiltration, angiogenesis, invasion, and migration. This review hopes to further discuss the importance of collagen in tumor metastasis through relevant research on collagen changes and tumor development and supplement or improve the understanding of the principles of colorectal cancer liver metastasis.
The classification and characteristics of collagen
Collagen is composed of 28 family members, each of which plays a unique role in the matrix structure [9]. Type I collagen is the main structural protein in the interstitial Extracellular Matrix (ECM) [10]. Type IV collagen is a crucial component of the Basement Membrane (BM), which is located on the basal surface of epithelial cells and endothelial cells and plays a vital role in tumor metastasis [11]. Collagen is composed of three peptide α chains, which can form either homotrimer or heterotrimer. The α chains also contain non-collagenous domains, which are removed by proteolysis in fibrillar collagens (e.g., I, II, III). After proteolysis, non-collagenous domains can exhibit new functions [12]. Research shows that changes in collagen proteins in the tumor microenvironment are associated with cancer metastasis and recurrence [13-15]. Type IV collagen is significantly upregulated in the tumor microenvironment of colorectal liver metastasis (CRLM) compared to normal liver tissue [16,17] and types I and III collagen are also densely expressed in the CRLM microenvironment [17]. Their molecular conformation also changes, with 19 out of 22 collagen α chains upregulated compared to normal tissue [16,18,19]. Meanwhile, under the action of matrix metalloproteinases (MMPs) [20,21], the activation of the fibrinolytic system and the degradation and remodeling of collagen fibers reshape the tumor microenvironment of liver metastases [16-19,21-23]. The histopathological growth pattern (HGP) model suggests that patients with a proliferative type of fibrosis characterized by collagen protein wrapping around liver metastases have a better prognosis [20,24-28]. However, Bram Piersma describes collagen as the “highway” for tumor metastasis, indicating that metastatic tumors can spread rapidly along collagen fibers [29]. From studies on breast cancer, it has been found that the orientation of collagen has a polarizing effect on tumor growth, as tumors tend to proliferate and metastasize parallel to collagen fibers but encounter obstacles in the vertical direction [30-32]. These studies suggest that the quantity, structure, orientation, and other characteristics of collagen may be related to tumor metastasis and prognosis.
Detection methods for collagen
Tumor proliferation can alter the function of collagen [15,33] and it has been shown that these changes depend on individual collagen levels [17]. Detecting collagen changes in blood or urine can reflect collagen dissolution and remodeling, but the exact location and specific reactions cannot be determined. Methods such as proteomics, which involve separating and extracting collagen for further testing, can reveal changes in collagen structure and quantity, but the conformation of collagen in a three-dimensional environment is lost. Immunohistochemistry methods, such as Van Gieson staining or Masson trichrome staining on pathological slides, can visually display the arrangement of collagen in the tumor microenvironment. However, they are influenced by factors such as staining time and batch, which prevents achieving quantitative analysis accuracy.
Collagen can generate second harmonic generation under conditions of multi-photon excitation due to its unique structure. These signals can be detected and used for multi-photon imaging [34-37]. Multi-photon imaging can visualize the assembly of collagen in tissue at the supramolecular level [38] and can be used to quantitatively extract collagen morphology, intensity, and texture features. Multi-photon imaging is an ideal method for collagen research. It does not require staining, is sensitive to collagen, and is fast and efficient. It can also be used for in vivo tissue imaging.
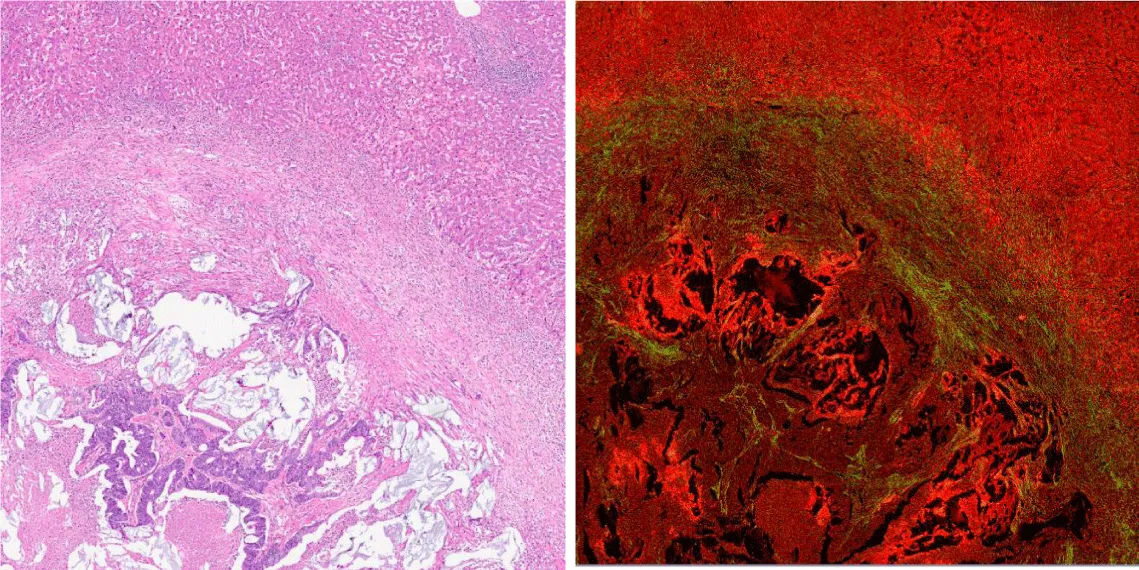
The two images are consecutive section scans of liver metastasis in colorectal cancer at the same location. The image on the left is HE staining, and the image on the right is a multiphoton imaging image. The bottom left corner of the image shows colorectal cancer tissue, while the top right corner shows liver cell tissue. In the multiphoton imaging, green represents collagen fibers (Figure 1).
The role of collagen in the pre-transfer microenvironment
Colorectal Cancer (CRC) exhibits extensive matrix collagen before liver metastasis. This process involves the formation of fibroblast-like cells and collagen fibers, as well as infiltration of endothelial cells, Hepatic Stellate Cells (HSC), and tumor cells. During this process, matrix collagen plays a role in the growth, matrix, and metastasis of tumor cells through autocrine or paracrine mechanisms [39]. For example, MMP-2, as an important receptor for matrix collagen, is regulated by fibroblasts, endothelial cells, and tumor cells [40]. The expression of MMP-2 on endothelial cells serves as a receptor for epithelial cell adhesion molecule (ECAM) and Epithelial Cell Adhesion-like molecule (ECAL) derived from tumor stem cells, while the epithelial cell adhesion molecule derived from tumor stem cells inhibits the expression of ECAM and ECAL through activation signaling pathways. Tumor cell matrix collagen is a major component of the connection between the extracellular matrix and endothelial cells [41]. Research has shown that Matrix Metalloproteinase 9 (MMP-9) can regulate metastatic behavior, including degradation of extracellular matrix components and formation of motile organelles, making it a therapeutic target for human colorectal tumors [42].
One fundamental stage during the metastasis process is the establishment of the Pre-Metastatic Niche (PMN) before the formation of clinically relevant metastasis, which plays a critical role in the occurrence of colorectal liver metastasis (CRCLM). Factors secreted by tumor and stromal cells induce the formation of the pre-metastatic niche by influencing the recruitment and functional activation of immune cells, leading to early changes in the microenvironment of distant organs without cancer cells, thus mediating the preparation of the metastatic site to facilitate subsequent tumor cell colonization [43]. The initiation of distant organ metastasis involves a gradual shift of tissue homeostasis towards a dysfunctional environment tilted towards the establishment of Circulating Tumor Cell (CTC) colonization: vascular leakage, lymphangiogenesis, extracellular matrix remodeling, and the generation of an immunosuppressive microenvironment [44]. Various cellular and molecular events are involved in the formation of liver Polymorphonuclear Cells (PMN) in CRC, such as Bone Marrow-Derived Cells (BMDCs), hepatic stellate cells, Kupffer cells, extracellular matrix, and CRC-derived factors. The formation of liver PMN depends on the complex interaction between CRC and the hepatic microenvironment, including recruitment of BMDCs, angiogenesis, immune suppression, inflammatory response, and remodeling of the extracellular matrix [44]. Exciting new research is beginning to reveal the complex interactions between multiple cell types and regulatory factors in the tumor microenvironment, where these cell types and regulatory factors cooperate to control the invasion and metastasis of tumor cells. By dynamically interacting with tumor cells, stromal cells participate in all stages of tumor initiation, development, metastasis, relapse, and drug response, thus influencing the fate of patients.
The migration and invasion of tumor cells in the liver are regulated by various factors, and matrix collagen is one important regulatory factor. Tumor cells induce the production of collagen fibers in the extracellular matrix by binding to collagen receptors in the surrounding matrix and then transmit these fibers to the liver tissue to promote the growth and metastasis of tumor cells by regulating angiogenesis and infiltration of tumor cells. Currently, many studies have shown a close correlation between matrix collagen and the migration and invasion of tumor cells.
The role of collagen in the dormancy of metastatic tumors
In 1993, COPPOCK et al. discovered that lung fibroblasts, after entering the resting phase (G0 phase), start expressing a series of quiescence-inducing genes (quiescins). The expression of these genes is crucial for maintaining the cells in a dormant state. They regulate and modulate various biological processes within the cells, including gene transcription, protein synthesis, and activity of intracellular signaling networks. The expression of these quiescence-inducing genes helps cells remain in a non-proliferative state and prevents them from entering the next stage of the cell cycle. The mechanism of cell entry into the resting phase holds significant importance in the study of lung fibroblasts and provides valuable insights into the development of pulmonary diseases and cellular biology research. Among them, 8 genes are upregulated, including decorin, C1r, Q6, Q10, and collagen chain genes COL6A1, COL3A1, COL1A1, and COL1A2. Particularly, the expression level of the COL3A1 gene reaches tenfold its original level after the cell enters the resting phase [45]. These research findings further suggest a hypothesis that collagen may contribute to maintaining a cell’s quiescent state, including tumor cells. At the same time, the transcriptional regulation of certain collagen genes in metastatic tumor cells may play a role in balancing proliferation and quiescence signals during the metastasis process. Metastatic tumor cells are in a non-proliferative quiescent state, called tumor dormancy, which can last for many years before regaining proliferative capacity [46]. Collagen is highly enriched in the extracellular matrix of dormant tumor cells and its composition differs from that of the proliferative phase extracellular matrix [47]. In the extracellular matrix of dormant tumor cells, tumor-derived COLIII expression increases, which is essential for maintaining the dormant state. Disruption of it restores tumor cell proliferation through DDR1-mediated STAT1 signaling.
Second Harmonic Generation (SHG) microscopy further revealed the changes in type III collagen structure and abundance accompanying the transition from dormancy to reactivation. Clinical sample analysis showed that the level of type III collagen was elevated in the tumors of patients with lymph node-negative head and neck squamous cell carcinoma compared to those with lymph node-positive metastasis. When COLIII disappeared in dormant cells, tumor cells regained their proliferative ability. Furthermore, collagen proteins are also involved in maintaining the quiescent state of other cell types such as stem cells. For example, COLV produced by muscle satellite stem cells maintains their quiescent state through the Calcitonin Receptor (CALCR), while COLVI regulates the self-renewal ability of satellite cells. Barkan et al. reviewed the potential contribution of the interaction between tumor cells and the microenvironment of metastatic sites in regulating tumor dormancy and growth, with a particular focus on the potential role of the extracellular matrix (ECM) in regulating dormancy maintenance and release. These long-term surviving disseminated tumor cells remain in a dormant state but may be triggered to proliferate by largely unknown factors [48].
The role of collagen in tumor metastasis
In the initial stages of tumorigenesis, tumorigenic epithelial cells proliferate rapidly in a two-dimensional (2D) environment at the top of the basal membrane [49,50]. As transformation progresses, cancer cells acquire the ability to penetrate the basal membrane and invade the underlying Extracellular Matrix (ECM) [50]. Consistent with the expression of an invasive phenotype, tumor cells adapt to the sudden transition in a growth environment. Invading cells are no longer confined to the planar surface of the ECM but are compelled to proliferate within a dense Three-Dimensional (3D) matrix primarily composed of type I collagen or cross-linked fibronectin [51,52]. However, despite the potent growth-inhibitory properties of the ECM [53-55], cancer cells can accelerate proliferation in this 3D environment by evading anti-growth signals [49,56].
The migration and invasion of tumor cells in the liver are regulated by multiple factors, and matrix collagen is one important regulatory factor among them. Tumor cells induce the production of collagen fibers in the extracellular matrix by binding to collagen receptors in the surrounding matrix, and then transmit these fibers to liver tissue, promoting the growth and metastasis of tumor cells by regulating angiogenesis and infiltration of tumor cells. Currently, many studies have already indicated a close correlation between matrix collagen and the migration and invasion of tumor cells.
Microenvironmental features associated with collagen architecture
In recent years, the focus of academic research has been on tumor immunity, and some new strategies for anti-tumor therapy have been developed. It has been found that the three-dimensional structure of the Extracellular Matrix (ECM) influences the migration of immune cells. When immune cells need to enter the interior of tumor tissue, they must actively migrate through the ECM. In the matrix of healthy tissues, the porosity of collagen fibers is relatively high, which is very beneficial for T lymphocytes and natural killer cells to enter tissues and play a physiological immune surveillance role. However, the size and density of collagen fibers can affect the external resistance encountered by immune cells during the migration process, further influencing the quantity and speed of immune cell entry into tissues. Specifically, the larger and denser the collagen fibers are, the greater the resistance faced by immune cells during migration, thereby limiting the quantity and speed of immune cell entry into tumor tissue.
These research findings are of great significance for us to better understand the process of tumor immunity and to seek ways to improve tumor treatment strategies. FRIEDL and other researchers found that immune cells’ nuclei can deform and move along loose and well-arranged collagen scaffolds in a typical amoeboid motion [57]. In addition, the Extracellular Matrix (ECM) plays a crucial role in regulating the immune response within primary tumors. ECM is a complex structural network composed of protein and sugar molecules that surround and provide support and protection to cells. During tumor development, changes occur in the ECM, resulting in the functional limitation of cytotoxic immune cells. When there is excessive expression of hyper-crosslinked collagen protein and glycoproteins surrounding cancer cells, the ECM’s hardness increases. This change in hardness hinders the infiltration and motility of immune cells. Immune cells are unable to effectively enter tumor tissue and interact with cancer cells. This phenomenon is known as “immune exclusion,” where immune cells are restricted around the tumor and unable to exert their cytotoxic effects.
Immune rejection is an important issue in tumor immunotherapy. During this process, tumor cells can evade attacks from the immune system by utilizing changes in the extracellular matrix (ECM), thus maintaining their survival and growth. Therefore, understanding and intervening in ECM changes are crucial for enhancing the effectiveness of immune therapy. In recent years, researchers have discovered some methods to overcome immune rejection. For example, they can use specific drugs or biotechnological means to reduce the stiffness of the ECM, thereby increasing the infiltration ability of immune cells. Additionally, they can restore the interaction between immune cells and cancer cells by targeting specific ECM molecules. By studying and understanding the role of ECM in tumor immunity, we can provide important clues for developing novel immunotherapies. These intervention strategies targeting ECM changes hold promise for providing more effective treatment options for cancer patients and opening new possibilities for future tumor immunotherapy. Research by HA RTMANN et al. found that although the expression of T cell chemokines CXCL10 or CXCL4 was upregulated in pancreatic cancer, the infiltration of T lymphocytes in the tumor was still suppressed due to the presence of high-density collagen [58]. SALMON et al. discovered that high-density collagen near blood vessels and tumor epithelial cells can guide the migration trajectory of T lymphocytes, limiting their entry into the tumor [59]. LARUE et al. also found that in pancreatic cancer, collagen synthesis and degradation directly influence the generation of a highly fibrotic and immune-suppressive microenvironment [60]. Tumor-associated Macrophages (TAMs) can increase intracellular arginine levels and upregulate inducible nitric oxide synthase through mannose receptor-mediated mechanisms, resulting in the synthesis of reactive nitrogen species (RNS). RNS, through a paracrine mechanism, stimulates pancreatic stellate cells to synthesize a large amount of collagen, which is then deposited in the extracellular matrix, leading to fibrosis in pancreatic tumors.
The prognostic value of collagen
Histopathological Growth Patterns (HGP) of liver metastases have become an important prognostic factor for the postoperative survival of colorectal cancer patients. The HGP occurs at the interface between tumor cells and the surrounding liver parenchyma, and it serves as a qualitative measure of tumor behavior. Previous studies have shown that different HGP types in colorectal cancer liver metastases reflect the interaction between the tumor and the host, and are associated with postoperative prognosis. HGP can be categorized into three main patterns: desmoplastic HGP, in which a thick matrix containing connective tissue, lymphocytes, and blood vessels separates the tumor from non-tumor parenchyma; pushing HGP, where tumor cells compress adjacent liver cell plates without invading them; and replacement HGP, where tumor components mimic the structure of liver cells and infiltrate into liver tissue. Two other HGP patterns, sinusoidal HGP and portal vein HGP, are extremely rare. Among these patterns, replacement HGP is associated with poorer overall survival, while desmoplastic HGP has the best prognosis [24,28]. Patients with desmoplastic HGP often experience intrahepatic recurrence, which has the best prognosis, while the other two types (non-desmoplastic HGP) are more likely to have multi-organ recurrence and poorer prognosis [61].
Recent research has shown that Colorectal Liver Metastases (CRLM) with Growth Patterns (HGP) characterized by desmoplastic reaction have higher rates of R0 resection. This suggests that the presence of a fibrous and inflammatory cell-rich stroma surrounding liver metastases can reduce positive margin rates. However, in the absence of such stroma, as in infiltrative or pushing HGP, the risk of positive margins and subsequent liver recurrence increases. In such cases, surgical resection should be expanded [62]. Invasive growth patterns and microvessel density (MVD) are associated factors, and primary tumors with high MVD tend to form liver metastases with high MVD (P = 0.007), which are predictive factors for poor prognosis in primary colorectal cancer (CRC) and colorectal liver metastases [26]. Histopathological growth patterns are associated with immune cell infiltration in patients with colorectal cancer liver metastases, as suggested by a Japanese study recently published online in 2023, indicating that desmoplastic HGP (dHGP) tumors have more CD68+ M1 macrophages and CD8+ lymphocytes at the tumor margin, which may be correlated with better postoperative survival in dHGP patients [63]. Therefore, accurate preoperative growth pattern prediction can help design more personalized surgical plans.
Summary and prospects
There are many hypotheses and speculations about the reasons for the changes in the tumor microenvironment, such as the seed and soil hypothesis, the co-evolution hypothesis, etc. However, regardless of how the tumor microenvironment is formed and changed, collagen, as the framework and main component of the microenvironment, can serve as a window to understanding the occurrence and development of tumors. By examining and classifying the collagen structure, it is possible to predict the possible biological behavior of tumors and even develop related targets to delay or limit tumor metastasis.
A series of studies have found a close association between tumor growth and the characteristics of collagen. In the case of in situ cancer, the hardness of collagen directly affects the stress of tumor cells, thereby promoting tumor growth. The hardness of collagen also affects the drainage function of microvessels and lymphatic vessels, leading to local hypoxia. Due to insufficient local vascular density, drugs cannot penetrate the tumor area smoothly. In addition, high collagen density hinders the migration of T cells, preventing them from contacting tumor cells. At the same time, cancer-associated fibroblasts (CAFs) traverse collagen, pulling tumor cells further for metastasis, and forming a high-speed channel. In the process of distant metastasis of the tumor, suitable collagen conditions are necessary for tumor colonization. Only with a suitable microenvironment can metastatic tumors settle and grow smoothly. In addition, the regulation of collagen can also induce dormancy in metastatic tumor tissue, thereby inhibiting further spread. In the microenvironment of metastatic tumors, collagen is closely related to factors such as angiogenesis and immune infiltration, forming different pathological histological growth patterns of metastatic tumor microenvironment. Collagen plays a crucial role in the process of tumor metastasis and becomes a potential therapeutic target. Research on collagen can cover aspects such as the prevention of tumor metastasis, treatment of dormant metastatic tumors, and treatment plans for metastatic tumors, highlighting the importance and diversity of its regulatory mechanisms.
The collagen in the tumor microenvironment is closely related to tumor behavior (such as metastasis, colonization, dormancy, etc.). Whether it is the hypothesis of tumor behavior following the tumor environment described in the “seed and soil” theory and “pre-metastatic microenvironment”, or the hypothesis of environmental changes following the tumor described in the “co-evolution” and “tumor-associated fibroblast induction” theories, both hypotheses are correct. What we can be sure of is that the tumor microenvironment collagen is closely related to tumor behavior. It can be used as a window to understand the past and future of tumors. In terms of short-term benefits, we can develop more precise surgical and post-operative monitoring plans for patients, and transform liver metastasis of colorectal cancer into a controllable long-term disease. In the long run, we can study the differences and mechanisms of collagen changes, use it as a target, and develop treatment methods that inhibit tumor budding and colonization, and promote tumor dormancy and immunity.
In summary, collagen can serve as an emerging target for investigating its association with the occurrence and development of colorectal cancer liver metastasis, playing a role in prevention, treatment, and surgical prognosis.
Authors’ contributions
Jiawen Fan and Dinghua Yang participated in the selection of the paper topic; Qing Zhu and Jianming Nie were responsible for literature retrieval; Jiawen Fan and Qing Zhu participated in the writing and revision of the paper. All authors have read and agreed to submit the final manuscript.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7-33. doi: 10.3322/caac.21708. Epub 2022 Jan 12. PMID: 35020204.
- Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E; ESMO Guidelines Committee. Electronic address: [email protected]. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023 Jan;34(1):10-32. doi: 10.1016/j.annonc.2022.10.003. Epub 2022 Oct 25. PMID: 36307056.
- Su YM, Liu W, Yan XL, Wang LJ, Liu M, Wang HW, Jin KM, Bao Q, Wang K, Li J, Xu D, Xing BC. Five-year survival post hepatectomy for colorectal liver metastases in a real-world Chinese cohort: Recurrence patterns and prediction for potential cure. Cancer Med. 2023 Apr;12(8):9559-9569. doi: 10.1002/cam4.5732. Epub 2023 Feb 27. PMID: 36846977; PMCID: PMC10166917.
- de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009 Sep;250(3):440-8. doi: 10.1097/SLA.0b013e3181b4539b. PMID: 19730175.
- Hashimoto M, Kobayashi T, Ishiyama K, Ide K, Ohira M, Tahara H, Kuroda S, Hamaoka M, Iwako H, Okimoto M, Ohdan H. Efficacy of repeat hepatectomy for recurrence following curative hepatectomy for colorectal liver metastases: A Retrospective Cohort Study of 128 patients. Int J Surg. 2016 Dec;36(Pt A):96-103. doi: 10.1016/j.ijsu.2016.10.004. Epub 2016 Oct 11. PMID: 27741421.
- Evrard S, Torzilli G, Caballero C, Bonhomme B. Parenchymal sparing surgery brings treatment of colorectal liver metastases into the precision medicine era. Eur J Cancer. 2018 Nov;104:195-200. doi: 10.1016/j.ejca.2018.09.030. Epub 2018 Oct 28. PMID: 30380461.
- Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg. 2016 Jan;263(1):146-52. doi: 10.1097/SLA.0000000000001194. PMID: 25775068.
- Liu M, Wang Y, Wang K, Bao Q, Wang H, Jin K, Liu W, Yan X, Xing B. Combined ablation and resection (CARe) for resectable colorectal cancer liver Metastases-A propensity score matching study. Eur J Surg Oncol. 2023 Sep;49(9):106931. doi: 10.1016/j.ejso.2023.05.006. Epub 2023 May 8. PMID: 37183048.
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011 Jan 1;3(1):a004978. doi: 10.1101/cshperspect.a004978. PMID: 21421911; PMCID: PMC3003457.
- Boot-Handford RP, Tuckwell DS. Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays. 2003 Feb;25(2):142-51. doi: 10.1002/bies.10230. PMID: 12539240.
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003 Jun;3(6):422-33. doi: 10.1038/nrc1094. PMID: 12778132.
- Egeblad M, Rasch MG, Weaver VM. The dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697-706. doi:10.1016/j.ceb.2010.08.015
- Martins Cavaco AC, Dâmaso S, Casimiro S, Costa L. Collagen biology making inroads into prognosis and treatment of cancer progression and metastasis. Cancer Metastasis Rev. 2020 Sep;39(3):603-623. doi: 10.1007/s10555-020-09888-5. PMID: 32447477.
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011 Mar;17(3):320-9. doi: 10.1038/nm.2328. PMID: 21383745; PMCID: PMC3569482.
- Provenzano PP, Inman DR, Eliceiri KW. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008; 6:11. doi:10.1186/1741-7015-6-11
- van Huizen NA, Coebergh van den Braak RRJ, Doukas M, Dekker LJM, IJzermans JNM, Luider TM. Up-regulation of collagen proteins in colorectal liver metastasis compared with normal liver tissue. J Biol Chem. 2019 Jan 4;294(1):281-289. doi: 10.1074/jbc.RA118.005087. Epub 2018 Nov 8. PMID: 30409905; PMCID: PMC6322866.
- Nyström H, Naredi P, Berglund A, Palmqvist R, Tavelin B, Sund M. Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumour. Anticancer Res. 2012 Dec;32(12):5183-91. PMID: 23225415.
- Murakami T, Kikuchi H, Ishimatsu H, Iino I, Hirotsu A, Matsumoto T, Ozaki Y, Kawabata T, Hiramatsu Y, Ohta M, Kamiya K, Fukushima M, Baba S, Kitagawa K, Kitagawa M, Konno H. Tenascin C in colorectal cancer stroma is a predictive marker for liver metastasis and is a potent target of miR-198 as identified by microRNA analysis. Br J Cancer. 2017 Oct 24;117(9):1360-1370. doi: 10.1038/bjc.2017.291. Epub 2017 Aug 24. PMID: 29065427; PMCID: PMC5672932.
- van Huizen NA, Burgers PC, van Rosmalen J, Doukas M, IJzermans JNM, Luider TM. Down-Regulation of Collagen Hydroxylation in Colorectal Liver Metastasis. Front Oncol. 2020 Sep 30;10:557737. doi: 10.3389/fonc.2020.557737. PMID: 33117689; PMCID: PMC7561380.
- Eefsen RL, Van den Eynden GG, Høyer-Hansen G, Brodt P, Laerum OD, Vermeulen PB, Christensen IJ, Wettergren A, Federspiel B, Willemoe GL, Vainer B, Osterlind K, Illemann M. Histopathological growth pattern, proteolysis and angiogenesis in chemonaive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:907971. doi: 10.1155/2012/907971. Epub 2012 Aug 2. PMID: 22919385; PMCID: PMC3419438.
- Illemann M, Bird N, Majeed A, Laerum OD, Lund LR, Danø K, Nielsen BS. Two distinct expression patterns of urokinase, urokinase receptor and plasminogen activator inhibitor-1 in colon cancer liver metastases. Int J Cancer. 2009 Apr 15;124(8):1860-70. doi: 10.1002/ijc.24166. PMID: 19123477.
- Nyström H. Extracellular matrix proteins in metastases to the liver - Composition, function, and potential applications. Semin Cancer Biol. 2021; 71:134-142. doi:10.1016/j.semcancer.2020.06.004
- Del Rio M, Mollevi C, Vezzio-Vie N, Bibeau F, Ychou M, Martineau P. Specific extracellular matrix remodeling signature of colon hepatic metastases. PLoS One. 2013 Sep 4;8(9):e74599. doi: 10.1371/journal.pone.0074599. PMID: 24023955; PMCID: PMC3762755.
- van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, Majeed AW, Eefsen RL, Coebergh van den Braak RRJ, Lazaris A, Fernandez MC, Galjart B, Laerum OD, Rayes R, Grünhagen DJ, Van de Paer M, Sucaet Y, Mudhar HS, Schvimer M, Nyström H, Kockx M, Bird NC, Vidal-Vanaclocha F, Metrakos P, Simoneau E, Verhoef C, Dirix LY, Van Laere S, Gao ZH, Brodt P, Reynolds AR, Vermeulen PB. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017 Nov 7;117(10):1427-1441. doi: 10.1038/bjc.2017.334. Epub 2017 Oct 5. PMID: 28982110; PMCID: PMC5680474.
- Van den Eynden GG, Majeed AW, Illemann M. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 2013; 73(7):2031-2043. doi:10.1158/0008-5472.CAN-12-3931
- Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY, Van Marck E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001 Oct;195(3):336-42. doi: 10.1002/path.966. PMID: 11673831.
- Nielsen K, Rolff HC, Eefsen RL, Vainer B. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol. 2014 Dec;27(12):1641-8. doi: 10.1038/modpathol.2014.4. Epub 2014 May 23. PMID: 24851832.
- Okano K, Yamamoto J, Kosuge T, Yamamoto S, Sakamoto M, Nakanishi Y, Hirohashi S. Fibrous pseudocapsule of metastatic liver tumors from colorectal carcinoma. Clinicopathologic study of 152 first resection cases. Cancer. 2000 Jul 15;89(2):267-75. PMID: 10918155.
- Piersma B, Hayward MK, Weaver VM. Fibrosis and cancer: A strained relationship. Biochim Biophys Acta Rev Cancer. 2020 Apr;1873(2):188356. doi: 10.1016/j.bbcan.2020.188356. Epub 2020 Mar 5. PMID: 32147542; PMCID: PMC7733542.
- Han W, Chen S, Yuan W, Fan Q, Tian J, Wang X, Chen L, Zhang X, Wei W, Liu R, Qu J, Jiao Y, Austin RH, Liu L. Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):11208-11213. doi: 10.1073/pnas.1610347113. Epub 2016 Sep 23. PMID: 27663743; PMCID: PMC5056065.
- Conklin MW, Gangnon RE, Sprague BL, Van Gemert L, Hampton JM, Eliceiri KW, Bredfeldt JS, Liu Y, Surachaicharn N, Newcomb PA, Friedl A, Keely PJ, Trentham-Dietz A. Collagen Alignment as a Predictor of Recurrence after Ductal Carcinoma In Situ. Cancer Epidemiol Biomarkers Prev. 2018 Feb;27(2):138-145. doi: 10.1158/1055-9965.EPI-17-0720. Epub 2017 Nov 15. PMID: 29141852; PMCID: PMC5809285.
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011 Mar;178(3):1221-32. doi: 10.1016/j.ajpath.2010.11.076. PMID: 21356373; PMCID: PMC3070581.
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013 Nov;19(11):1423-37. doi: 10.1038/nm.3394. PMID: 24202395; PMCID: PMC3954707.
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003 Nov;21(11):1369-77. doi: 10.1038/nbt899. PMID: 14595365.
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007 Mar 15;67(6):2649-56. doi: 10.1158/0008-5472.CAN-06-1823. PMID: 17363585.
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7075-80. doi: 10.1073/pnas.0832308100. Epub 2003 May 19. PMID: 12756303; PMCID: PMC165832.
- Campagnola P. Second harmonic generation imaging microscopy: applications to diseases diagnostics. Anal Chem. 2011 May 1;83(9):3224-31. doi: 10.1021/ac1032325. Epub 2011 Mar 29. PMID: 21446646; PMCID: PMC3104727.
- Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc. 2012 Mar 8;7(4):654-69. doi: 10.1038/nprot.2012.009. PMID: 22402635; PMCID: PMC4337962.
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997 Sep 3;89(17):1260-70. doi: 10.1093/jnci/89.17.1260. PMID: 9293916.
- Oshima T, Kunisaki C, Yoshihara K, Yamada R, Yamamoto N, Sato T, Makino H, Yamagishi S, Nagano Y, Fujii S, Shiozawa M, Akaike M, Wada N, Rino Y, Masuda M, Tanaka K, Imada T. Clinicopathological significance of the gene expression of matrix metalloproteinases and reversion-inducing cysteine-rich protein with Kazal motifs in patients with colorectal cancer: MMP-2 gene expression is a useful predictor of liver metastasis from colorectal cancer. Oncol Rep. 2008 May;19(5):1285-91. PMID: 18425389.
- Asano T, Tada M, Cheng S, Takemoto N, Kuramae T, Abe M, Takahashi O, Miyamoto M, Hamada J, Moriuchi T, Kondo S. Prognostic values of matrix metalloproteinase family expression in human colorectal carcinoma. J Surg Res. 2008 May 1;146(1):32-42. doi: 10.1016/j.jss.2007.02.011. Epub 2007 May 31. PMID: 17543340.
- Lubbe WJ, Zhou ZY, Fu W, Zuzga D, Schulz S, Fridman R, Muschel RJ, Waldman SA, Pitari GM. Tumor epithelial cell matrix metalloproteinase 9 is a target for antimetastatic therapy in colorectal cancer. Clin Cancer Res. 2006 Mar 15;12(6):1876-82. doi: 10.1158/1078-0432.CCR-05-2686. PMID: 16551873.
- Yofe I, Shami T, Cohen N, Landsberger T, Sheban F, Stoler-Barak L, Yalin A, Phan TS, Li B, Monteran L, Scharff Y, Giladi A, Elbaz M, David E, Gurevich-Shapiro A, Gur C, Shulman Z, Erez N, Amit I. Spatial and Temporal Mapping of Breast Cancer Lung Metastases Identify TREM2 Macrophages as Regulators of the Metastatic Boundary. Cancer Discov. 2023 Dec 12;13(12):2610-2631. doi: 10.1158/2159-8290.CD-23-0299. PMID: 37756565.
- Yang L, Li T, Shi H, Zhou Z, Huang Z, Lei X. The cellular and molecular components involved in pre-metastatic niche formation in colorectal cancer liver metastasis. Expert Rev Gastroenterol Hepatol. 2021 Apr;15(4):389-399. doi: 10.1080/17474124.2021.1848543. Epub 2020 Nov 30. PMID: 33174441.
- Coppock DL, Kopman C, Scandalis S, Gilleran S. Preferential gene expression in quiescent human lung fibroblasts. Cell Growth Differ. 1993 Jun;4(6):483-93. PMID: 8396966.
- Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer. 2020 Jul;1(7):672-680. doi: 10.1038/s43018-020-0088-5. Epub 2020 Jul 6. PMID: 33681821; PMCID: PMC7929485.
- Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, Naba A, Aguirre-Ghiso JA, Bravo-Cordero JJ. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2022 Jan;3(1):90-107. doi: 10.1038/s43018-021-00291-9. Epub 2021 Dec 13. PMID: 35121989; PMCID: PMC8818089.
- Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, Green JE. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010 Jul 15;70(14):5706-16. doi: 10.1158/0008-5472.CAN-09-2356. Epub 2010 Jun 22. PMID: 20570886; PMCID: PMC3436125.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57-70. doi: 10.1016/s0092-8674(00)81683-9. PMID: 10647931.
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375-379. doi:10.1038/35077241
- Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998 Oct 30;95(3):365-77. doi: 10.1016/s0092-8674(00)81768-7. PMID: 9814707.
- Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001; 93(3):178-193. doi:10.1093/jnci/93.3.178
- Nishiyama T, Tsunenaga M, Nakayama Y, Adachi E, Hayashi T. Growth rate of human fibroblasts is repressed by the culture within reconstituted collagen matrix but not by the culture on the matrix. Matrix. 1989 Jun;9(3):193-9. doi: 10.1016/s0934-8832(89)80050-2. PMID: 2789331.
- Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996 Dec 13;87(6):1069-78. doi: 10.1016/s0092-8674(00)81801-2. PMID: 8978611.
- Henriet P, Zhong ZD, Brooks PC, Weinberg KI, DeClerck YA. Contact with fibrillar collagen inhibits melanoma cell proliferation by up-regulating p27KIP1. Proc Natl Acad Sci U S A. 2000 Aug 29;97(18):10026-31. doi: 10.1073/pnas.170290997. PMID: 10944199; PMCID: PMC27660.
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002 Aug;2(8):563-72. doi: 10.1038/nrc865. PMID: 12154349.
- Friedl P, Entschladen F, Conrad C, Niggemann B, Zänker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998 Aug;28(8):2331-43. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. PMID: 9710211.
- Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014 Jul 1;20(13):3422-33. doi: 10.1158/1078-0432.CCR-13-2972. Epub 2014 Apr 24. PMID: 24763614.
- Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012 Mar;122(3):899-910. doi: 10.1172/JCI45817. Epub 2012 Feb 1. PMID: 22293174; PMCID: PMC3287213.
- LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2119168119. doi: 10.1073/pnas.2119168119. Epub 2022 Apr 11. PMID: 35412885; PMCID: PMC9169723.
- Galjart B, Nierop PMH, van der Stok EP, van den Braak RRJC, Höppener DJ, Daelemans S, Dirix LY, Verhoef C, Vermeulen PB, Grünhagen DJ. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019 May;22(2):355-368. doi: 10.1007/s10456-019-09661-5. Epub 2019 Jan 12. PMID: 30637550; PMCID: PMC6475515.
- Viganò L, Branciforte B, Laurenti V, Costa G, Procopio F, Cimino M, Del Fabbro D, Di Tommaso L, Torzilli G. The Histopathological Growth Pattern of Colorectal Liver Metastases Impacts Local Recurrence Risk and the Adequate Width of the Surgical Margin. Ann Surg Oncol. 2022 Sep;29(9):5515-5524. doi: 10.1245/s10434-022-11717-8. Epub 2022 Jun 10. PMID: 35687176.
- Kanno H, Hisaka T, Fujiyoshi K, Akiba J, Hashimoto K, Fujita F, Akagi Y. Prognostic Significance of the Histopathological Growth Pattern and Tumor-Infiltrating Lymphocytes in Stratifying Survival After Hepatectomy for Colorectal Liver Metastases. Ann Surg Oncol. 2023 May;30(5):3139-3147. doi: 10.1245/s10434-022-12905-2. Epub 2022 Dec 15. PMID: 36520232.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
