Annals of Cytology and Pathology
The Sentient Propagation-Reactive B Cell-Rich Lymphoid Proliferation
Consultant Histopathologist, Histopathology, Panjab University/A.B. Diagnostics, India
Author and article information
Cite this as
Bajaj A. The Sentient Propagation-Reactive B Cell-Rich Lymphoid Proliferation. Ann Cytol Pathol. 2024; 9(1): 020-024. Available from: 10.17352/acp.000032
Copyright License
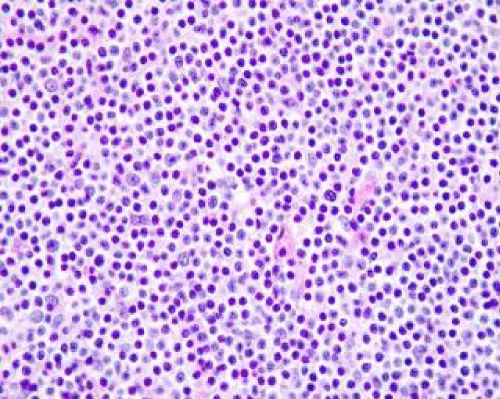
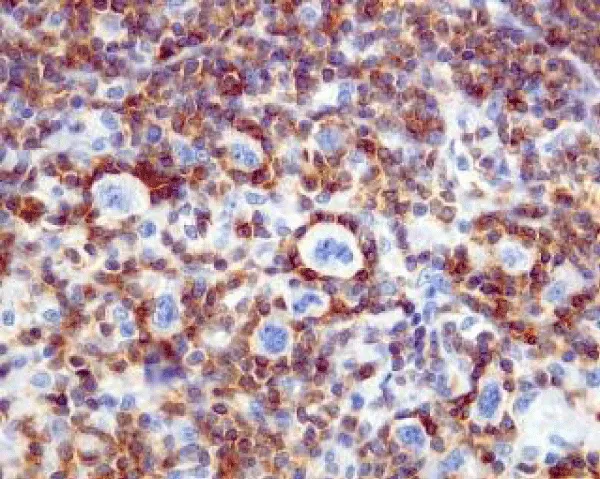
© 2024 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Reactive B cell-rich lymphoid proliferation is a heterogeneous group of non-neoplastic lymphoid cell proliferation recapitulating diverse B cell lymphomas. The lesion is commonly categorized as nodal and extra-nodal follicular proliferations, nodal and extra-nodal nodular proliferations or nodal and extra-nodal immunoblastic proliferations. Florid follicular hyperplasia is comprised of quantifiably enhanced, disseminated primary and secondary lymphoid follicles with irregular outlines. Hyperplastic Germinal Centres (GCs) are comprised of admixed centroblasts and centrocytes, reactive T cells, Follicular Dendritic Cells (FDCs) and tingible body macrophages. Lymph node architecture depicts lymphoid follicles comprised of cells expressing B cell antigens wherein primary follicles are pre-eminently constituted of small lymphocytes BCL2+, BCL6- and CD10- immune reactivity. Progressive Transformation of Germinal Centre (PTGC) is constituted of singular or few enlarged lymphoid follicles of 4x to 5x magnitude wherein mantle zone cells display extensive invagination into adjacent germinal centres. Hyaline Vascular Castleman’s Disease (HVCD) delineates innumerable lymphoid follicles confined to the lymph node cortex and medulla or diverse extramedullary sites.
Reactive B cell-rich lymphoid proliferation emerges as a heterogeneous group of non-neoplastic lymphoid cell proliferations which simulate diverse B cell lymphomas. Commonly, lymphoid tissue within various lymph nodes or extra-nodal sites is implicated. Generally, the heterogeneous, non-neoplastic lymphoid proliferations display distinctive patterns as follows:
~nodal and extra-nodal follicular proliferation.
~nodal and extra-nodal nodular proliferations.
~nodal and extra-nodal immunoblastic proliferation.
Follicular or nodular proliferations confined to regional lymph nodes as
Florid follicular hyperplasia is comprised of quantifiably enhanced, disseminated primary and secondary lymphoid follicles with irregular outlines and variable magnitude. Florid lesions may extend into the medulla. Hyperplastic Germinal Centres (GCs) are comprised of admixed centroblasts and centrocytes, reactive T cells, Follicular Dendritic Cells (FDCs) and tingible body macrophages. Mitotic activity is significant. B lymphocytes emerge as centroblasts preponderantly confined to the dark zone of the germinal centre. Centroblasts are impregnated with enlarged vesicular nuclei with one to three peripheral nucleoli and delineate 3x to 4x the magnitude of small lymphocytes [1,2]. Centrocytes are predominantly confined to the light zone of the germinal centre and appear as miniature to intermediate cells demonstrating cleaved, hyperchromatic nuclei and miniature to absent nucleoli (Figure 1 & 2). The centroblasts and centrocytes are immune reactive to BCL6+, CD10+, LMO2+, HGAL+ GCET(centre)+ and OCT2+. Constituent T lymphocytes are spherical, miniature cells immune reactive to CD3+ and BCL2+. A subset of T helper cells (TFH) demonstrate immune reactive PD-1 / CD279+, BCL6+, CD10+, CXCL13+, ICOS+ and polarized CD4+ [1,2]. Follicular dendritic cells (FDCs) are few (~1%) and are pervaded with elongated cytoplasmic processes, dual, square adjacent nuclei (kissing cells), vesicular nuclear chromatin and miniature nucleolus. Cells are immune reactive to CD21+, CD23+ or CD35+. Tingible body macrophages are permeated with abundant, pale cytoplasm with karyorrhectic, ovoid or twisted vesicular nuclei. The prominence of tingible body macrophages induces a ‘starry sky’ countenance to the lesion. Cells appear immune reactive to CD4+, CD68+ or CD163+ [1,2]. Well-developed mantle zones necessitate demarcation from germinal centres are preponderantly composed of small lymphocytes and IgD+ lymphocytic cells. Cellular extension beyond the lymph node capsule into circumscribing perinodal soft tissue is minimal to absent. Localization of aforesaid nodules may indicate concomitant disease wherein extended evaluation is mandated for diagnostic confirmation [1,2]. Specific disease processes emerge as ~cervical lymph nodes may be implicated by infectious mononucleosis ~posterior cervical lymph nodes may display toxoplasmosis ~parotid, submaxillary or epitrochlear lymph nodes may expound infection with human immunodeficiency virus (HIV) ~cervical and axillary lymph nodes may depict cat scratch disease or dermatopathic lymphadenitis ~inguinal lymph nodes may be involved with sexually transmitted diseases [2,3]. Lymph node architecture depicts lymphoid follicles comprised of cells expressing B cell antigens. Primary follicles are pre-eminently constituted of small lymphocytes which display an immune reactive panel as BCL2+, BCL6- and CD10-. Ki-67 proliferative index is <10%. Secondary follicles demonstrate germinal centres which are immune and non-reactive to BCL2- [2,3]. T Follicular Helper (TFH) cells configure a subset of T cells confined to germinal centres which are BCL2+ and may occasionally increase. The aforesaid lesion may simulate follicular lymphoma. The paediatric subtype of Follicular Lymphoma (FL) appears BCL2- although germinal centre cells appear FOXP1+. Ki-67 proliferative index is elevated. Focal polarization with centroblast-rich dark zones and centrocyte-rich pale zones may appear [2,3]. The decimated Ki-67 proliferative index confined to germinal centres appears anomalous and non-indicative of follicular lymphoma. Lymphocytes immune reactive to BCL6+ and CD10+ are confined to germinal centres and appear minimally distributed within inter-follicular areas [2,3]. Flow cytometry depicts polytypic B cells as CD10+ with absent T cell antigens. Polymerase chain reaction (PCR) expounds polyclonal immunoglobulin heavy chain genetic rearrangements. Chromosomal translocation t(14;18)(q32;q21) or IGH::BCL2 genetic fusion appears absent [3,4].
Progressive Transformation of Germinal Centre (PTGC) is constituted of singular or few enlarged lymphoid follicles of 4x to 5x magnitude wherein mantle zone cells display extensive invagination into adjacent germinal centres. Intervening lymph node parenchyma expounds Reactive Follicular Hyperplasia (RFH). The lesion is devoid of rosette formation by PD-1+ T Follicular Helper (TFH) cells circumscribing enlarged B cells. Tingible body macrophages are infrequently encountered within germinal centres [3,4]. Morphological alterations are gradual and comprised of ~ early-stage disease with hyperplastic germinal centres wherein fusion of germinal centres within a singular follicle of magnitude 2x to 3x is observed. Mantle zone small B lymphocytes display intrinsic migration into the germinal centre ~delayed stages delineate dissolution of germinal centres with the consequent occurrence of islands of or singularly scattered centrocytes and centroblasts admixed with follicular dendritic cells within mantle zone small B lymphocytes. Preservation of B cell and T cell compartments and prominent follicular pattern is encountered. Germinal centre and mantle zone lymphocytes expound pan-B cell antigens [3,4]. Mantle zone cells demonstrate an immunological profile as BCL2+, IgD+, BCL6- and CD10-. Disrupted germinal centres are pervaded with cells displaying BCL6+, CD10+, BCL2- and IgD- immunological profiles. Immune staining with CD21, CD23 or CD35 expresses disruption of Follicular Dendritic Cell (FDCs) meshwork. Up to 50% of lymph nodes delineate a population of IgG4+ plasma cells [4,5]. Flow cytometric analysis demonstrates a predominant population of polytypic B cells. Genetic assays expound polyclonal genomic rearrangements within the IGH gene [4,5].
Hyaline Vascular Castleman’s Disease (HVCD) commonly occurs in young adults < 30 years although the paediatric population can be implicated. Tumefaction delineates innumerable lymphoid follicles confined to the lymph node cortex and medulla or diverse extramedullary sites. Morphological features such as obliteration of sub-capsular and inter-follicular sinuses and ≥ 2 germinal centres within a follicle or ‘twinning’ may be observed [5,6]. Characteristically, lymphoid follicles are enlarged and impregnated with regressed germinal centres with involution. Lymphoid follicles are preponderantly composed of Follicular Dendritic Cells (FDCs) with few lymphocytes. Constituent follicular dendritic cells may be hyperplastic or dysplastic. Several follicles articulate ‘lollipop’ outlines [5,6]. Mantle zones appear prominent. Rings and concentric accumulation of mantle zone lymphocytes may configure an ‘onion skin’ appearance. Inter-follicular or stromal components appear distinct. A predominant stromal component may depict occasional hyaline vascular follicles. Clusters of plasmacytoid dendritic cells may be discerned although a paucity of plasma cells and immunoblasts is encountered. Alternatively, cellular components are abundantly disseminated within the plasma cell variant of Castleman’s disease. Sclerotic vascular articulations traverse and are radially disseminated into the germinal centres. Quantifiably enhanced high endothelial venules with hyalinised walls are discernible [6,7]. Tumour cells appear as polytypic B cells, T cells and plasma cells which are immune and non-reactive to human herpes virus 8 (HHV8-). An enhanced population of follicular dendritic cells (FDCs) concurrent with the involution of germinal centres may occur. Tumour cells appear immune reactive to CD21+, CD23+, CD35+ or epidermal growth factor receptor (EGFR+). Dysplastic Follicular Dendritic Cells (FDCs) appear variably immune reactive to diverse immune markers about FDCs. Hyaline vascular Castleman’s disease requires segregation from neoplasms as classic Hodgkin’s lymphoma concurrent with Castleman’s disease and Castleman’s disease-like follicles confined to various infectious lymphadenopathies [6,7].
Nodal immunoblastic proliferation as infectious mononucleosis may be engendered by acute Epstein-Barr virus (EBV) infection. Morphological alterations are variable, contingent to duration of disease and appear as ~early stage comprised of reactive lymphoid follicles and minimal para-cortical expansion ~progressive stage comprised of minimal lymphoid follicles along with significant expansion of inter-follicular tissue ~advanced stage comprised of effacement of lymphoid follicles with prominent inter-follicular expansion ~inter-follicular areas with innumerable immunoblasts or a ‘mixed’ inflammatory infiltrate. Scattered or abundant immunoblasts may be discerned. Constituent lymphocytes vary from small to intermediate or enlarged cells. A variable component of plasma cells, plasmacytoid cells and histiocytic cells with infrequent eosinophils are encountered [6,7]. Lymph node sinuses are uniformly impregnated with immunoblasts, monocytoid B cells and histiocytic cells. Bi-nucleated immunoblasts may simulate Hodgkin’s Reed Sternberg (HRS)-like cells. Preserved lymph node architecture demonstrates commingled B cells, T cells and immunoblasts, a population which is indicative of a reactive process. Focal necrosis may be discerned. Hodgkin’s Reed Sternberg (HRS)-like cells appear immune reactive to CD45+, CD30+ and non-reactive to CD15- [6,7]. Epstein Barr virus (EBV) latency type 3 may be demonstrated by EBV encoded RNAs (EBER) within innumerable infected cells as small lymphocytes, intermediate lymphocytes or Hodgkin’s Reed Sternberg (HRS)-like immunoblasts. Tumour cells appear immune reactive to EBNA1+, EBNA3+, LMP1+, LMP2+ and variably immune reactive to EBNA2+. Constituent lymphocytes appear immune reactive to CD8+ and T cell intracellular antigen 1 (TIA1+) [7,8].
Herpesvirus lymphadenitis is configured as an infrequently discerned complication arising due to primary exposure or reactivation of herpesvirus infection. Lesion simulates infectious mononucleosis and depicts pre-eminent immunoblastic hyperplasia of the paracortex. Tumefaction delineates well-circumscribed areas of necrosis and sinus histiocytosis. Multinucleated cells pervaded with ground glass nuclear chromatin and haloed, intra-nuclear viral inclusions appear to abut foci of necrosis. The occurrence of Herpes Simplex Virus (HSV) may be confirmed with cogent immunohistochemistry [7,8].
Cytomegalovirus lymphadenitis demonstrates morphological features concordant with lymph node lesions occurring due to diverse viral infections. Frequently, monocytoid B cell hyperplasia may be encountered. Constituent lymphoid cells display characteristic ‘owl-eyed’ centric, intra-nuclear inclusions with a distinctive halo. Commonly, T lymphocytes and endothelial cells may be infected with Cytomegalovirus (CMV). Cogent immunohistochemistry may be beneficially applied for appropriate discernment of Cytomegalovirus (CMV) [8,9].
Extra-nodal follicular proliferation is constituted of
Florid reactive lymphoid hyperplasia wherein the majority of lesions are represented as reactive tertiary lymphoid tissue and appear concurrent with an infectious agent, autoimmune phenomena or chronic repetitive trauma. Aforesaid contributory factors may induce extra-nodal marginal zone lymphoma with organisms designated as ~Helicobacter pylori which induce gastric lesions ~Borrelia burgdorferi which induce cutaneous lesions ~Chlamydia psittaci which engender conjunctival lesions ~Chlamydia trachomatis which engenders lesions of the cervix ~Campylobacter jejuni which induces lesions confined to small intestine ~Achromobacter xylosoxidans which induces lesions confined to pulmonary parenchyma. Besides, disorders such as extrahepatic biliary obstruction, Hashimoto’s thyroiditis or Sjögren’s syndrome may ensue. Segregation of florid reactive lymphoid hyperplasia from marginal zone lymphoma may be challenging. Additionally, cutaneous reactive lymphoid hyperplasia necessitates demarcation from primary cutaneous follicular centre lymphoma [9,10]. Florid reactive Lymphoid hyperplasia of the female reproductive tract commonly implicates the cervix followed in frequency by the endometrial cavity and vulva. The majority of lesions delineate superficial dissemination of chronic inflammatory cells as lymphoid infiltrates along with or devoid of mucosal ulceration [9,10]. Of Variable architecture, tumefaction depicts patchy, nodular or diffuse lesions wherein the majority of lesions display secondary follicles. Florid reactive lymphoid hyperplasia appears to recapitulate normal lymph node architecture and demonstrates distinct organization of B follicular and T inter-follicular compartments [10,11]. Cytological assessment delineates a component of small lymphocytes and polytypic plasma cells admixed with quantifiable variable granulocytes and histiocytic cells. The majority of lesions display a component of enlarged immunoblasts immune reactive to CD30+ disseminated within the cellular constituents. Clonal IGH genetic rearrangements may be commonly observed [11,12] (Table 1).
Extra-nodal immunoblastic proliferations are constituted of ~Epstein Barr virus (EBV)+ mucocutaneous ulcer which emerges as a lymphoproliferative disorder typically composed of enlarged B cells infected by EBV. Besides, Reed Sternberg-like (RS-like) cells may be observed. Subjects appear to lack systemic symptoms, lymphadenopathy, hepatosplenomegaly or bone marrow lesions. Commonly, oral mucosa is implicated followed in frequency by cutaneous lesions. Miniature, multiple lesions may ensue within a singular anatomic region [11,12]. Upon microscopy, a well-circumscribed, superficial ulcer with a subjacent polymorphous inflammatory infiltrate is encountered. The ulcer has a sharply defined, deep-seated base encompassed by an intense rim of small lymphocytes. Foci of vascular invasion are common. Ki-67 proliferation index appears elevated. An estimated ~33% of instances depict monoclonal IGH or TCR genetic rearrangements. Hodgkin’s Reed Sternberg-like cells appear immune reactive to CD45+ or CD30+ and immune non-reactive to CD15- [12,13]. Epstein Barr virus latency type 3 may be detected by EBV encoded RNAs (EBER) within innumerable infected cells as miniature to intermediate lymphocytes and Hodgkin’s Reed Sternberg (HRS)-like immunoblasts. Tumour cells appear immune reactive to LMP1+ and EBNA2+. The basal perimeter of the ulcer is rimmed by CD8+ T cells [12,13].
Conclusion
Nodal immunoblastic proliferation as infectious mononucleosis engendered by acute Epstein Barr virus (EBV) infection demonstrate morphological alterations as early stage comprised of reactive lymphoid follicles and minimal para-cortical expansion, progressive stage comprised of minimal lymphoid follicles along with significant expansion of inter-follicular tissue, advanced stage comprised of effacement of lymphoid follicles with prominent inter-follicular expansion and inter-follicular areas with innumerable immunoblasts or a ‘mixed’ inflammatory infiltrate. Herpesvirus lymphadenitis depicts pre-eminent immunoblastic hyperplasia of the paracortex. Cytomegalovirus lymphadenitis frequently demonstrates monocytoid B cell hyperplasia. Extra-nodal follicular proliferations are constituted of florid reactive lymphoid hyperplasia wherein the majority of lesions represent reactive tertiary lymphoid tissue. Extra-nodal immunoblastic proliferations are constituted of Epstein Barr virus (EBV)+ mucocutaneous ulcer which emerges as a lymphoproliferative disorder typically composed of enlarged B cells infected by EBV.
- Ferry JA, Hill B, Hsi ED. Mature B, T and NK-cell, plasma cell and histiocytic/dendritic cell neoplasms: classification according to the World Health Organization and International Consensus Classification. J Hematol Oncol. 2024;17(1):51. Available from: https://pubmed.ncbi.nlm.nih.gov/38978094/
- Oikonomidi C, Troupi M, Marinos L, Liatsos D, Chrysikos D, Filippou D, et al. Cutaneous B-cell Pseudolymphoma: A Rare Case Masquerading a Thoracic Mass in a Fourteen-Year-Old Male Patient. Cureus. 2023;15(4):e38003. Available from: https://pubmed.ncbi.nlm.nih.gov/37223157/
- Hooper MJ, Veon FL, LeWitt TM, Chung C, Choi J, Zhou XA, et al. Cutaneous T-Cell-Rich Lymphoid Infiltrates After SARS-CoV-2 Vaccination. JAMA Dermatol. 2022;158(9):1073-1076. Available from: https://pubmed.ncbi.nlm.nih.gov/35857292/
- Mintoff D, Scerri L, Betts A. SARS-CoV-2 mRNA vaccine injection site pseudolymphoma. J Eur Acad Dermatol Venereol. 2022;36(1):e20-e22. Available from: https://pubmed.ncbi.nlm.nih.gov/34547136/
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma-A review on the spectrum and a proposal for a new classification. J Cutan Pathol. 2020;47(1):76-97. Available from: https://pubmed.ncbi.nlm.nih.gov/31237707/
- Tian Z, Shiyu Z, Tao W, Li L, Yuehua L, Hongzhong J. Lymphoma or pseudolymphoma: A report of six cases and review of the literature. Dermatol Ther. 2019;32(4):e12807. Available from: https://pubmed.ncbi.nlm.nih.gov/30589489/
- Miguel D, Peckruhn M, Elsner P. Treatment of Cutaneous Pseudolymphoma: A Systematic Review. Acta Derm Venereol. 2018 Mar 13;98(3):310-317. Available from: https://pubmed.ncbi.nlm.nih.gov/29136262/
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190(13):E398. Available from: https://pubmed.ncbi.nlm.nih.gov/29615424/
- Romero-Pérez D, Blanes Martínez M, Encabo-Durán B. Cutaneous Pseudolymphomas. Actas Dermosifiliogr. 2016;107(8):640-51. English, Spanish. Available from: https://pubmed.ncbi.nlm.nih.gov/27289134/
- Besch-Stokes JG, Patel MH, Brumfiel CM, Costello CM, Rule W, Rosenthal A, et al. Cutaneous B cell pseudolymphoma treated with rituximab and methotrexate. Dermatol Online J. 2021;27(9). Available from: https://pubmed.ncbi.nlm.nih.gov/34755980/
- Fan Y, Thong BKS, Binshen O, Shen X, Yi H, Wang C. Non-neoplastic B-cell predominant lymphoid proliferations at the organs exposed to external environment mimicking lymphoma: A potential diagnostic pitfall. Int J Immunopathol Pharmacol. 2024:3946320241264369. Available from: https://pubmed.ncbi.nlm.nih.gov/38886178/
- Pupier G, Sautès-Fridman C. B cells! Don't go the wrong way in this tumor. Immunity. 2024;57(7):1454-1456. Available from: https://pubmed.ncbi.nlm.nih.gov/38986440/
- Evans MG, Brynes RK, Crymes A, Reid J, Haghighi N, Botros C, et al. Role of immunoglobulin heavy and light chain gene rearrangement analysis in differentiating between benign and malignant bone marrow B-cell lymphoid aggregates. Hum Pathol. 2022;130:58-64. Available from: https://pubmed.ncbi.nlm.nih.gov/36252861/
- Image 1 and 2 Courtesy: Pathology outlines. PathologyOutlines.com [Internet]. Available from: https://www.pathologyoutlines.com/
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
