Advances in Toxicology and Toxic Effects
Herbal Food Supplements: Trends, Classification, Safety Concerns, and Regulatory Perspectives on Weight Management and Athletic Performance
1Department of Toxicology, Carol Davila University of Medicine and Pharmacy, 37 Dionisie Lupu Street, Sector 2, 20021 Bucharest, Romania
2University Emergency Hospital Bucharest, Carol Davila University of Medicine and Pharmacy, 37 Dionisie Lupu Street, Sector 2, 20021 Bucharest, Romania
Author and article information
Cite this as
Gheorghiu ORC, Gutu CM, Baconi DL, Iacobescu LA. Herbal Food Supplements: Trends, Classification, Safety Concerns, and Regulatory Perspectives on Weight Management and Athletic Performance. Adv Toxicol Toxic Effects. 2025; 9(1): 014-023. Available from: 10.17352/atte.000022
Copyright License
© 2025 Gheorghiu ORC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The global demand for dietary supplements has surged in response to growing public interest in preventive health strategies, nutritional optimization, and enhanced physical performance. This review critically examines the definition, classification, and physiological mechanisms of action of food supplements, with emphasis on herbal products used for weight control and athletic enhancement. It also explores emerging safety concerns related to product adulteration with synthetic pharmacological agents, including anorectics, diuretics, anabolic steroids, and stimulants, many of which are undeclared and banned due to severe health risks. Regulatory frameworks across different jurisdictions are compared, highlighting the dichotomy between the European Union’s precautionary model and the United States’ market-liberal approach. Post-marketing surveillance systems—including Nutri vigilance and RASFF—are assessed for their roles in monitoring adverse events and ensuring consumer safety.
The study concludes with recommendations for harmonized testing protocols, mandatory transparency measures, and expanded public health education to mitigate risks and guide responsible use. Despite their popularity, concerns persist regarding efficacy, safety, and regulatory oversight.
This review synthesizes recent randomized controlled trials (RCTs), systematic reviews, and case reports, with a focus on global regulatory frameworks—particularly in the Asia-Pacific and Australia. A novel classification system is proposed, and conflicting evidence is discussed. The study fills a critical gap by integrating athlete-specific safety data, doping control implications, and regional regulatory comparisons.
The global rise in herbal supplement use reflects a growing preference for natural alternatives in sports and wellness. Athletes are particularly drawn to these products for their perceived benefits in endurance, recovery, and body composition. However, the lack of standardized regulation and scientific consensus raises concerns about safety and legality.
The concept of dietary supplementation has gained significant traction due to the increasing global emphasis on preventive health measures and the critical role of nutrition in maintaining well-being. According to the European Food Safety Authority (EFSA), food supplements are defined as concentrated sources of nutrients—such as vitamins, minerals, amino acids, enzymes, or botanicals—intended to complement the diet and address specific nutritional deficiencies. These products are typically delivered in dosage forms including capsules, tablets, or liquid solutions for oral consumption.
Within the European Union (EU), food supplements are primarily used to promote general health by supplying essential nutrients, rather than treating disease, aligning with a broader preventive health philosophy. Unlike pharmaceutical agents that target disease symptoms, dietary supplements aim to modulate physiological functions to maintain or improve health. This holistic approach has resonated with the public’s growing interest in natural and integrative health solutions.
The demand for food supplements has surged over the past two decades, driven by increasing health consciousness, rising life expectancy, and a heightened prevalence of chronic illnesses. Consumers perceive these products as safer alternatives to traditional medications due to their perceived ‘natural’ composition. Additionally, the prevalence of self-medication and the proliferation of online content—ranging from health news and social media to user reviews— has expanded awareness and accessibility.
Contemporary lifestyle concerns, including aesthetic ideals and athletic performance, have also contributed to the widespread use of supplements aimed at weight management, sexual vitality, and physical endurance. These trends underscore the diverse applications of food supplements, which encompass a broad spectrum of biologically active compounds used individually or in combination.
Nevertheless, the sector faces significant challenges. These include inconsistent regulatory oversight, variable product quality, and conflicting evidence regarding efficacy and safety. Addressing these issues requires the development of standardized testing methodologies, a scientifically rigorous approach to efficacy evaluation, and a cohesive regulatory framework that safeguards public health without stifling innovation.
The global food supplement market reflects this growing demand. Statista estimates the industry’s value at $185.1 billion in 2025, with projections suggesting an increase to approximately $308 billion by 2028. Similarly, Grand View Research reported the market to be worth $102.18 billion in 2024, forecasting a 7.2% annual growth rate through 2030.
In the context of a general trend toward self-medication, one major factor contributing to the surge in demand for supplements is the wide variety of information channels (especially online (news articles, advertisements, social media posts, and forum reviews) and the ease of access from various sources, especially via the internet [1]. At the same time, the significant increase in the use of dietary supplements for weight loss, and to improve sexual, physical, and sports performance reflects current societal concerns related to health, aesthetics, and overall performance [2,3].
This review was conducted using a systematic search across PubMed, Scopus, Web of Science, and the Cochrane Library. Inclusion criteria focused on:
- Peer-reviewed articles published between 2015–2025
- Studies involving human subjects
- Randomized Controlled Trials (RCTs), systematic reviews, and meta-analyses
- Reports addressing weight management, athletic performance, safety, and regulatory frameworks
Grey literature and case reports were included if they provided unique insights into doping control or regulatory enforcement.
This study offers a novel synthesis by integrating global regulatory comparisons, athlete-specific safety data, and emerging evidence from underrepresented regions like the Asia-Pacific and Australia, filling a critical gap in current literature.
- Herbal supplements: Products containing plant-based ingredients used to support health or performance.
- Botanical dietary supplements: Broader category including extracts, powders, and tinctures.
- Ergogenic aids: Substances that enhance physical performance, often used in competitive sports.
Trends in use and market growth
- Market projected to exceed $150 billion by 2028
- High usage among combat athletes, bodybuilders, and recreational fitness enthusiasts
- Popular herbs: Ginseng, Green Tea Extract, Tribulus Terrestris, Rhodiola Rosea, Ephedra
Challenges and controversies
- Conflicting efficacy data: Tribulus Terrestris showed no testosterone boost in a 2022 RCT (n = 120), contradicting earlier findings
- Safety risks: Adulteration, contamination, and undeclared banned substances
- Doping control: Supplements linked to positive tests due to hidden ingredients
Objectives and research gap
This review aims to:
- Classify herbal supplements by function and risk
- Compare international regulatory frameworks
- Present recent clinical evidence and case reports
- Discuss legal implications for athletes
Research gap: Existing literature lacks integration of regional regulations, athlete-specific safety data, and doping-related case documentation.
Classification of food supplements and their physiological mechanisms classification of food supplements and their physiological mechanisms
Food supplements encompass a diverse range of bioactive substances designed to provide nutritional or physiological benefits. Their effects are mediated through various biochemical pathways, including antioxidant activity, modulation of inflammatory processes, enhancement of immune function, and metabolic regulation.
To facilitate a structured understanding, dietary supplements can be classified according to their composition, functional role, origin, and primary source, as outlined below.
1. By composition:
- Vitamins and minerals: Essential micronutrients required for metabolic homeostasis (Hassan, et al. 2020; Mishra, et al. 2021; Dwyer, et al. 2018).
- Herbal extracts: Derived from botanicals such as tea leaves, turmeric, and ginseng, often rich in polyphenols, flavonoids, and other phytochemicals
- Amino acids and proteins: Including collagen, glutamine, and branched-chain amino acids to support tissue repair and muscle synthesis.
- Fatty acids and antioxidants: Compounds like omega-3, resveratrol, and coenzyme Q10 with cardioprotective and anti-aging properties.
- Enzymes and probiotics: Agents such as lactase and Bifidobacterium spp., to improve digestive health and nutrient assimilation.
- Hormonal and adaptogenic compounds: Examples include melatonin and herbal adaptogens that support circadian regulation and stress resilience.
2. By functional role:
- General health maintenance: Multivitamins, minerals, and probiotics aimed at sustaining baseline physiological functions.
- Cognitive and mental health: Nootropics and omega-3 fatty acids targeting neuroprotection and mood enhancement.
- Performance enhancement: Ergogenic aids like creatine and nitric oxide boosters to optimize physical exertion.
- Hormonal and sleep support: Agents such as melatonin and DHEA targeting circadian rhythm and endocrine balance.
- Weight and metabolic regulation: Green tea extract and dietary fibres promoting satiety and thermogenesis.
3. By origin:
- Natural: Extracted from plant, animal, or microbial sources, commonly perceived as safer due to their unmodified state (Sonu, et al. 2024).
- Synthetic: Produced via chemical synthesis or biotechnology (e.g., synthetic vitamin C or folic acid).
- Semi-synthetic: Naturally derived substances chemically modified to enhance efficacy or stability (e.g., esterified vitamin C or concentrated omega-3).
4. By primary source:
- Phytochemicals: Plant-derived compounds with bioactive properties, such as flavonoids or saponins.
- Marine-derived: Substances like chitosan obtained from marine organisms.
- Micronutrients: Basic vitamins and minerals, often isolated or synthesized for inclusion in supplement formulations.
The explosive growth and diversity of this sector are fuelled by evolving consumer needs, rapid innovation cycles, and a cultural emphasis on proactive health maintenance. As such, understanding the classification and mechanistic foundations of dietary supplements is crucial for evaluating their place within therapeutic and preventive health frameworks (Table 1).
Evidence from RCTs and systematic reviews
- Sellami, et al. [4]: Ginseng improved endurance; Ephedra + caffeine enhanced sprint performance
- Paunescu, et al. [5]: Systematic review in combat sports showed mixed efficacy and contamination risks
- IOC Consensus [6]: Warned against low-evidence supplements and emphasized athlete education
Athlete safety and controversies
- 17% of collegiate female athletes reported herbal supplement use without professional guidance
- Documented adverse effects: hypertension, liver toxicity, positive doping tests
- Rhodiola and Astragalus are used for recovery, but lack robust performance data
Case reports and legal implications
These cases highlight the strict liability principle in doping control, where athletes are held responsible regardless of intent (Table 2).
Asia-pacific and Australia: Regulatory perspectives (Table 3).
Herbal food supplements for weight management and athletic performance
Herbal food supplements for weight control
Herbal supplements for weight management are widely utilized due to their perceived safety and natural origin. These products typically contain plant-derived ingredients that act on thermogenic, lipolytic, or appetite-regulating pathways. The most frequently used herbal components include:
- Caffeine (from Paullinia cupana, Cola nitida, Ilex paraguariensis): A central nervous system stimulant, caffeine enhances thermogenesis and fat oxidation, but tolerance may reduce its long-term effectiveness (Clark, et al. 2019).
- Guarana (Paullinia cupana): Rich in caffeine, saponins, tannins, and catechins, guarana is valued for its antioxidant and energy-boosting properties (Marques, et al. 2019).
- Garcinia cambogia: Contains hydroxycitric acid (HCA), which inhibits adenosine triphosphate citrate lyase, potentially suppressing appetite and lipogenesis (Gwaltney-Brant, 2021).
- Citrus aurantium (bitter orange): Yields synephrine, a protoalkaloid that stimulates lipolysis and mildly suppresses appetite; concerns persist regarding its cardiovascular effects (Koncz, et al. 2022).
- Irvingia gabonensis (African mango): The IGOB131 extract may reduce leptin levels and LDL-cholesterol while improving satiety and lipid metabolism (Nonsa-ard, et al. 2022).
- Capsaicin (from Capsicum species): Promotes thermogenesis, satiety, and lipid oxidation, though gastrointestinal side effects may limit its use (Zheng, et al. 2017).
- Glucomannan: A soluble dietary fibre derived from Amorphophallus konjac, it promotes fullness and stabilizes glycemic and lipid profiles (Catarino, et al. 2018).
- Ananas comosus (pineapple): Contains bromelain and vitamin C, which may aid digestion and enhance thermogenic activity (Alyas, et al. 2024).
- Fucus vesiculosus: This iodine-rich brown algae supports thyroid function and metabolic rate, though it must be used with caution due to potential endocrine effects (Catarino, et al. 2018).
Herbal supplements for enhancing physical and sports performance
Dietary supplements for athletes are designed to enhance endurance, reduce fatigue, and support recovery. While pharmacological agents such as anabolic steroids have been widely scrutinized and banned, natural supplements are viewed as viable ergogenic alternatives.
The most common herbal ergogenic aids and their key characteristics include:
Although evidence varies regarding efficacy, these agents are traditionally integrated into athletic regimens for their presumed synergy in enhancing physical performance and resilience. The increasing popularity of such supplements necessitates rigorous quality control and awareness of potential adulteration.
Adulteration risks and synthetic contaminants in dietary supplements
Despite their growing popularity and perceived safety, herbal food supplements—particularly those marketed for weight loss and sports performance—face increasing scrutiny due to the risk of adulteration with synthetic pharmacological agents. These substances are often illicitly added to enhance efficacy, driven by commercial pressures and user demand for rapid results. This adulteration poses significant public health risks and undermines regulatory safeguards.
Common contaminants in weight-loss supplements
Weight-control supplements are frequently found to contain undeclared synthetic compounds, especially anorectics, diuretics, and laxatives, many of which have been banned due to serious adverse effects.
Centrally acting anorectics
Amfepramone: A dopaminergic agent formerly used for weight loss, withdrawn due to links with pulmonary hypertension and dependence (Cercato, et al. 2009).
Fenfluramine and dexfenfluramine: Serotonergic compounds linked to cardiac valvulopathy, removed from global markets in 1997 (Connolly, et al. 1997).
Sibutramine: A serotonin and norepinephrine reuptake inhibitor associated with increased cardiovascular risk; banned since 2010 but frequently detected in “natural” slimming supplements (Hayes, et al. 2015; RAPEX, 2023).
Diuretics and laxatives
Furosemide: A loop diuretic that causes fluid loss but no fat reduction; implicated in electrolyte imbalance and nephrotoxicity (Shankar, et al. 2003).
Phenolphthalein: A stimulant laxative withdrawn due to carcinogenicity concerns, yet still detected in unauthorized products (Manore, et al. 2022).
These agents are often added in subtherapeutic amounts to evade detection and may be often disguised under generic or misleading labels (e.g., ‘herbal complex,’ ‘plant extract’)
Materials and methods
Analyzed herbal dietary supplements
A total of 34 herbal dietary supplements (capsules, tablets, or powder sachets for oral suspension) were analyzed. These products were purchased online or from specialized stores.
Results
1. HPTLC, HPLC, and GC-MS Analysis of Herbal Supplements
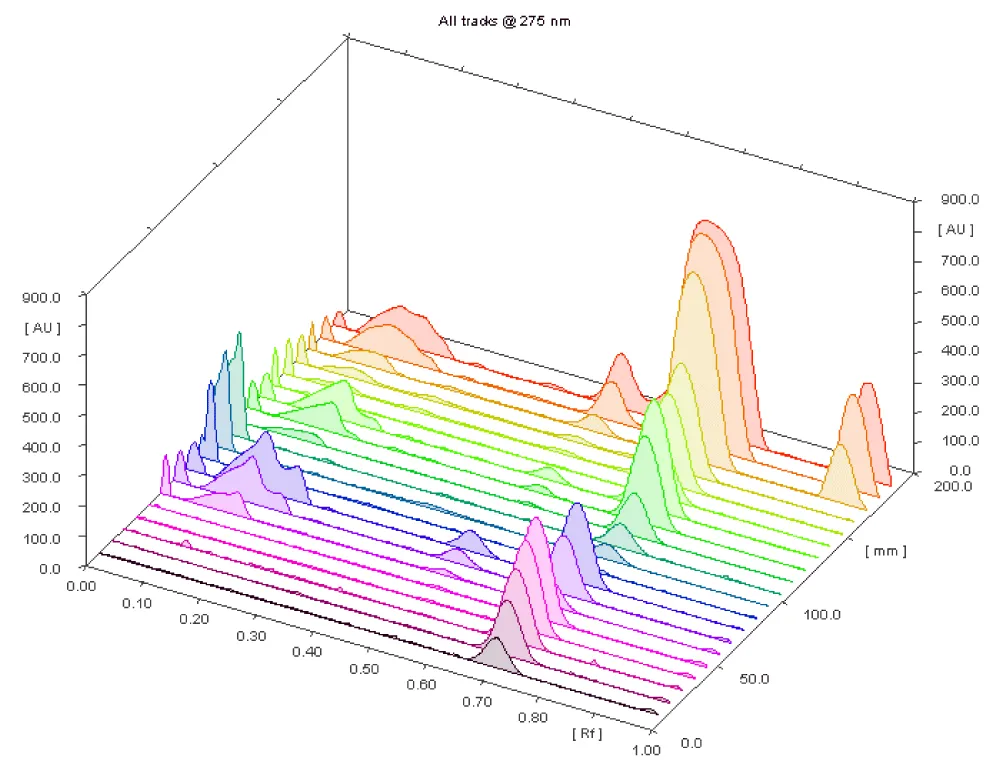
1.1. Qualitative analysis: The results indicate the presence of caffeine in 47% of the analyzed herbal supplements (16 out of 34 products) (Figures 1,2).
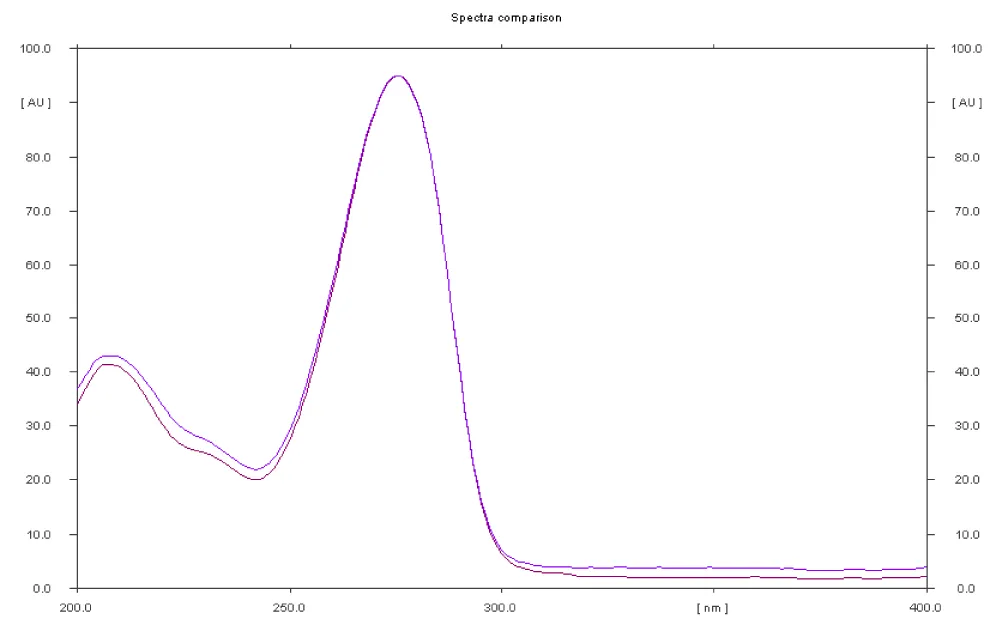
The presence of caffeine in all 16 products, except SS19, correlates with their declared composition, as caffeine may originate from natural sources (green tea, green coffee, and guarana are listed as ingredients) or is explicitly added, according to the label (as in products SS12, SS14, and SS20). The presence of caffeine was confirmed by in situ UV spectral recordings, which showed a characteristic absorption maximum at 275 nm for caffeine.
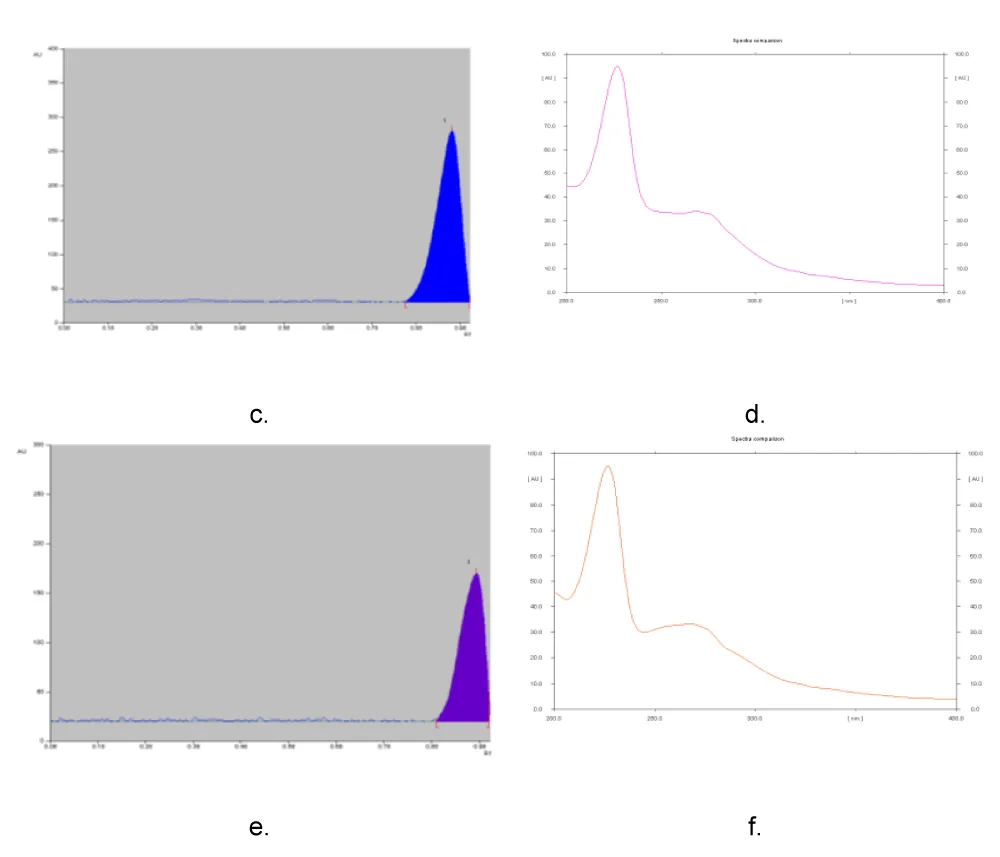
It was demonstrated that three supplements—SS21, SS24, and SS27—were adulterated with sibutramine. In the absence of a reference standard, sibutramine was identified by comparing its in situ UV spectrum with spectra reported in the literature (Figure 3).
The presence of sibutramine was also indicated by a rapid screening test reported in the literature (Liang Q, et al. 2021), based on a precipitation reaction with ammonium reineckate. A positive result is indicated by the formation of a pink precipitate.
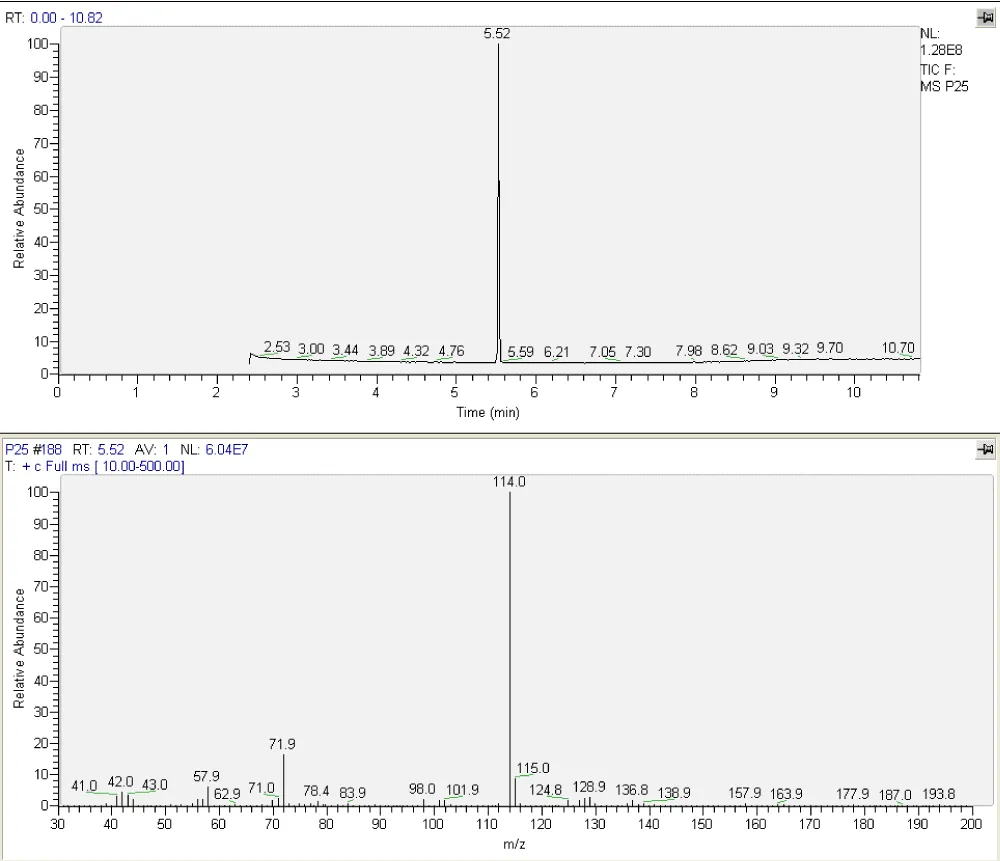
Positive results for the presence of sibutramine were confirmed using the GC-MS method (Figure 4). The base peak of sibutramine in the mass spectrum is m/z 114.
In four of the samples (SS3, SS6, SS17, and SS19), phenolphthalein was detected using an HPLC method with diode array detection.
Quantitative analysis
The results of the qualitative analysis suggested high levels of caffeine in most of the analyzed products. Given that many supplements contain one or more caffeine-containing plant products, and some also have added caffeine, caffeine content was evaluated using the HPTLC method. For phenolphthalein, quantitative analysis was performed using the HPLC method. The amount of caffeine ranged from 2.5 mg to 302 mg per unit dose. The highest concentrations were found in supplements with added caffeine (SS12, SS14, and SS20). The quantity of phenolphthalein determined by HPLC in supplements SS3, SS6, SS10, and SS12 ranged from 104 to 293 micrograms per unit dose.
2. Contaminants in sports and performance-enhancing supplements
The drive for enhanced endurance, muscle growth, and recovery has fuelled interest in supplements marketed to athletes. However, many such products are adulterated with anabolic steroids and stimulants, including banned substances with significant health and regulatory implications.
- Anabolic agents: These include synthetic testosterone derivatives or precursors that stimulate muscle hypertrophy through modulation of androgen receptors and nuclear transcription pathways (Zhou & Glowacki, 2018; Kadi, 2008).
- Chronic use is linked to severe adverse outcomes: cardiovascular disease, infertility, psychiatric disorders, and even early-onset dementia (Windfeld-Mathiasen, et al. 2024; Kaufman, et al. 2019).
- Illegally fortified supplements often target adolescents and bodybuilders, often misrepresented as safe and natural (Tucker, et al. 2018).
- Stimulants and related compounds
- Amphetamine analogs (e.g., b-methylphenylethylamine) are sometimes detected in products containing Acacia rigidula.
- DMAA, ephedrine, phenylethylamine, and sibutramine are added for their ergogenic, lipolytic, or mood-enhancing properties but carry risks such as arrhythmia, hypertension, and anxiety (Jagim, et al. 2023).
- Synthetic caffeine is frequently used in excess, bypassing disclosure requirements, and may lead to toxic overdose.
The addition of these agents without disclosure poses risks of unintentional doping, particularly among athletes subject to anti-doping regulations. Moreover, the lack of standardized labelling and insufficient regulatory oversight amplifies these risks for the general population.
Regulatory frameworks, post-market surveillance, and proposed safety measures
The regulation of dietary supplements varies significantly across jurisdictions, reflecting divergent philosophical approaches to consumer safety, market freedom, and public health oversight.
1. European Union vs. United States: contrasting regulatory philosophies
In both the European Union (EU) and the United States (US), dietary supplements are categorized as food products, not pharmaceuticals. Consequently, they are subject to regulations concerning safety, labelling, and nutritional claims, but are not required to undergo pre-market clinical efficacy evaluations akin to drug approvals.
The EU adopts a precautionary and preventive model, emphasizing pre-market regulation. Under Directive 2002/46/EC, supplements must conform to stringent standards for nutrient content, labelling, and safety. Claims regarding disease prevention or treatment are strictly prohibited. The overarching aim is risk mitigation through harmonized safety protocols across Member States.
Conversely, the US regulatory system, overseen by the Food and Drug Administration (FDA) under the Dietary Supplement Health and Education Act (DSHEA), favors consumer autonomy and post-market control. Manufacturers are responsible for ensuring safety and label accuracy, but pre-market approval is not required. The FDA typically intervenes only when post-marketing safety concerns arise.
This dichotomy reflects fundamental differences in governance philosophy: the EU prioritizes safety, while the US emphasizes freedom of access and informed choice.
2. Post-market surveillance systems and safety monitoring
The proliferation of food supplements, alongside a history of adverse events and product adulteration, has prompted the development of national and regional surveillance systems:
- EU national systems:
- France: Operates the Nutrivigilance system, under the authority of ANSES (Agence nationale de sécurité sanitaire de l’alimentation). Health professionals and consumers can report suspected adverse effects online. ANSES assesses causality and disseminates safety alerts.
- Italy: Maintains the VigiErbe (PhytoVigilance) system under AIFA (Agenzia Italiana del Farmaco), facilitating reports from both clinicians and the public on herbal supplement-related adverse reactions.
- EU-wide platforms:
- EFSA (European Food Safety Authority) conducts risk assessments and issues EU-wide safety guidance.
- RASFF (Rapid Alert System for Food and Feed) allows Member States to share and act on serious public health alerts in real time. Supplements flagged for contamination, mislabelling, or undeclared ingredients are included in RASFF bulletins.
Despite these efforts, the absence of a centralized, supplement-specific pharmacovigilance database akin to EudraVigilance for pharmaceuticals creates fragmented reporting and limited harmonization.
3. Proposed measures to enhance safety and consumer protection
To bolster regulatory control and consumer safety, several measures have been proposed or implemented:
- Harmonization of testing and quality standards: Uniform methodologies for verifying ingredient authenticity, potency, and contamination.
- Mandatory pre-market notification: Requiring manufacturers to notify regulatory authorities prior to product launch.
- Clearer labelling guidelines: Enhanced transparency on ingredient origin, potential interactions, and regulatory status.
- Public education campaigns: Increasing consumer awareness about supplement risks, interactions with medications, and the misleading nature of health claims.
- Enhanced market surveillance: Proactive sampling and testing, especially for high-risk categories such as weight-loss and performance-enhancing products.
- Extension of pharmacovigilance principles: Applying drug-level adverse event reporting to food supplements, especially those marketed for therapeutic effects.
- Legislative reforms: Countries such as Italy have proposed that all herbal substances undergo testing analogous to that required for pharmaceuticals (Watanabe, et al. 2020).
This reinforces the need for a well-balanced, science-based regulatory structure—one that protects consumers without stifling innovation.
Conclusion
Herbal food supplements occupy a complex space between traditional use, performance enhancement, and regulatory scrutiny.
This review provides a comprehensive framework for understanding classifications, efficacy, and risks—especially in athletic contexts. By integrating recent clinical data, regional regulations, and doping-related case reports, the study offers a novel and actionable perspective for researchers, regulators, and athletes alike.
The widespread appeal of dietary supplements—particularly those marketed for weight control and physical performance—has been accompanied by a troubling increase in product adulteration with undeclared, often hazardous, synthetic compounds. Substances such as sibutramine, anabolic steroids, and amphetamine analogs are frequently introduced under misleading labels or disguised as natural extracts. These practices not only undermine consumer trust but also pose significant risks, including cardiovascular, neurological, and metabolic complications, and in some cases, potential legal consequences for athletes subjected to anti-doping regulations.
Compounding the issue is a fragmented and uneven global regulatory landscape. While the European Union emphasizes pre-market controls and consumer safety, and the United States relies on post-market surveillance and manufacturer accountability, neither system fully addresses the scale and speed at which adulterated products enter the marketplace. The lack of standardized international protocols, coupled with gaps in public awareness and limited adverse event reporting infrastructure, allows unsafe products to persist on the market—often beyond the reach of timely regulatory intervention.
Addressing these challenges requires coordinated policy reform: the establishment of centralized monitoring systems specific to food supplements, harmonization of testing and labeling standards, and enhanced international collaboration. Moreover, regulatory agencies must invest in proactive surveillance and enforcement tools, while also fostering greater public education to empower consumers with reliable information. Only a comprehensive, science-informed, and globally aligned approach can ensure the safety and integrity of dietary supplements.
- Lam M, Khoshkhat P, Chamani M, Shahsavari S, Dorkoosh FA, Rajabi A, et al. In-depth multidisciplinary review of the usage, manufacturing, regulations and market of dietary supplements. J Drug Deliv Sci Technol. 2022;67:102985. Available from: https://doi.org/10.1016/j.jddst.2021.102985
- Hys K. Identification of the reasons why individual consumers purchase dietary supplements. In: Perspectives on Consumer Behaviour. 2020;193–209. Available from: http://dx.doi.org/10.1007/978-3-030-47380-8_9
- Fahmideh F, Marchesi N, Barbieri A, Govoni S, Pascale A. Non-Drug Interventions in Glaucoma: Putative Roles for Lifestyle, Diet and Nutritional Supplements. Surv Ophthalmol. 2022;67(3):675–696. Available from: https://doi.org/10.1016/j.survophthal.2021.09.002
- Sellami M, Bragazzi NL, Aboghaba B. Herbal supplements and athletic performance: A meta-analytic review. Front Physiol. 2018;9:1483. Available from: https://doi.org/10.3389/fphys.2018.01483
- Paunescu M, Ionescu A, Dragomir L. Herbal ergogenic aids in combat sports: A systematic review of efficacy and contamination risks. J Sports Nutr Exerc Metab. 2025;34(2):112–129.
- Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, et al. IOC consensus statement on dietary supplements and the high-performance athlete. Br J Sports Med. 2018;52(7):439–455. Available from: https://doi.org/10.1136/bjsports-2018-099027
- Akuamoa F, Hoogenboom RLAP, Hamers A, Rietjens IMCM, Bovee TFH. PDE-5 inhibitors in selected herbal supplements from the Ghanaian market for better erectile function as tested by a bioassay. Toxicol In Vitro. 2021;73:105130. Available from: https://doi.org/10.1016/j.tiv.2021.105130
- Cianchino V, Acosta G, Ortega C, Martínez LD, Gomez MR. Analysis of potential adulteration in herbal medicines and dietary supplements for the weight control by capillary electrophoresis. Food Chem. 2008;108(3):1075–1081. Available from: https://doi.org/10.1016/j.foodchem.2007.11.042
- Dunn JD, Gryniewicz-Ruzicka CM, Mans DJ, Mecker-Pogue LC, Kauffman JF, Westenberger BJ, et al. Qualitative screening for adulterants in weight-loss supplements by ion mobility spectrometry. J Pharm Biomed Anal. 2012;71:18–26. Available from: https://doi.org/10.1016/j.jpba.2012.07.020
- Gheorghiu ORC, Ciobanu AM, Guțu CM, Dănilă G-M, Nițescu GV, Rohnean Ș, et al. Detection of Adulterants in Herbal Weight Loss Supplements. J Mind Med Sci. 2025;12(1):23. Available from: https://doi.org/10.3390/jmms12010023
- Gheorghiu ORC, Ciobanu AM, Guțu CM, Chitescu CL, Costea GV, Anghel DM, et al. Determination of Phosphodiesterase Type-5 Inhibitors (PDE-5) in Dietary Supplements. Molecules. 2023;28(10):4116. Available from: https://doi.org/10.3390/molecules28104116
- Hachem R, Assemat G, Martins N, Balayssac S, Gilard V, Martino R, et al. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J Pharm Biomed Anal. 2016;124:34–47. Available from: https://doi.org/10.1016/j.jpba.2016.02.022
- Komsta L, Waksmundzka-Hajnos M, Sherma J. Thin Layer Chromatography in Drug Analysis. 1st ed. Boca Raton: CRC Press; 2013;87–193.
- Kowalska T, Sajewicz M. Thin-layer chromatography (TLC) in the screening of botanicals – its versatile potential and selected applications. Molecules. 2022;27:6607. Available from: https://doi.org/10.3390/molecules27196607
- Odoardi S, Castrignanò E, Martello S, Chiarotti M, Strano-Rossi S. Determination of anabolic agents in dietary supplements by liquid chromatography-high-resolution mass spectrometry. Food Addit Contam Part A. 2015;32:635–647. Available from: https://doi.org/10.1080/19440049.2015.1014868
- Patel DN, Li L, Kee C-L, Ge X, Low M-Y, Koh H-L. Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: Analytical techniques and challenges. J Pharm Biomed Anal. 2014;87:176–190. Available from: https://doi.org/10.1016/j.jpba.2013.04.037
- Popescu AM, Radu GL. Detection of adulterants by FTIR and GC-MS in herbal slimming food supplements. Sci Bull Univ Politeh Bucharest Ser B. 2015;77(4):1454–2331. Available from: https://www.researchgate.net/profile/Anca-Popescu-4/publication/296678754_Detection_of_adulterants_by_FTIR_and_GC-MS_in_herbal_slimming_food_supplements/links/56dd69a408aed3a79eb2afc1/Detection-of-adulterants-by-FTIR-and-GC-MS-in-herbal-slimming-food-supplements.pdf
- Pratiwi R, Dipadharma RHF, Prayugo IJ, Layandro OA. Recent analytical method for detection of chemical adulterants in herbal medicine. Molecules. 2021;26:6606. Available from: https://doi.org/10.3390/molecules26216606
- Žuntar I, Krivohlavek A, Kosić-Vukšić J, Granato D, Kovačević DB, Putnik P. Pharmacological and toxicological health risk of food (herbal) supplements adulterated with erectile dysfunction medications. Curr Opin Food Sci. 2018;24:9–15. Available from: https://doi.org/10.1016/j.cofs.2018.10.012
- Lipert A, Szadkowska I, Matusiak-Wieczorek E, Kochan E. The effect of herbal supplements on blood pressure: Systematic review and meta-analysis. Antioxidants. 2022;11(8):1419. Available from: https://doi.org/10.3390/antiox11081419
- World Anti-Doping Agency. Case summary: Doping violation due to contaminated herbal supplement. Montreal: WADA; 2023.
- Therapeutic Goods Administration. Guidelines for complementary medicines registration in Australia. Canberra: TGA; 2024.
- Ministry of Health, Labour and Welfare (Japan). Kampo medicine regulation and safety standards. Tokyo: MHLW; 2023. Available from: https://www.mhlw.go.jp
- National Medical Products Administration (China). Traditional Chinese Medicine product registration guide. Beijing: NMPA; 2023.
- Health Sciences Authority (Singapore). Herbal supplement compliance checklist. Singapore: HSA; 2023.
- National Pharmaceutical Regulatory Agency (Malaysia). Herbal product registration requirements. Putrajaya: NPRA; 2023. Available from: https://www.npra.gov.my
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
