Global Journal of Allergy
Stevens-Johnson syndrome due to Lamotrigine
Silvio Espínola*
Cite this as
Espínola S. Stevens-Johnson syndrome due to Lamotrigine. Glob J Allergy. 2024;10(1):001-005. Available from: 10.17352/2455-8141.000027Copyright License
© 2024 Espínola S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Lamotrigine is an anticonvulsant drug that has been widely used to treat epilepsy, as a mood stabilizer (in cases of bipolar type 1 disorder), and in the management of neuropathic pain; It is used both in monotherapy and in complementary therapy. Considered a relatively new medication, approved by the Food and Drug Administration in 1994, its benefits include a greater margin of safety compared to other anticonvulsants; However, it causes serious adverse skin reactions, such as Stevens-Johnson syndrome. Approximately 8% of patients receiving lamotrigine develop a benign maculopapular rash during the first 4 months of treatment. A case of Stevens-Johnson syndrome caused by the drug is presented and a review of the condition and the probable pathways that trigger this delayed hypersensitivity immune response is carried out.
Clinical case
A case is presented of a patient who has been known to be epileptic since she was 11 years old, treated with valproic acid 250 mg, presenting 3 episodes of seizures per year.
As a family pathological history, she reports that the mother, the mother’s sister, and a brother are epileptic [1-3].
At 21 years of age, she began to have more frequent seizures, tremors, and bradylalia, so valproate was suspended and lacosamide 300 mg daily and topiramate 100 mg daily were added to the treatment.
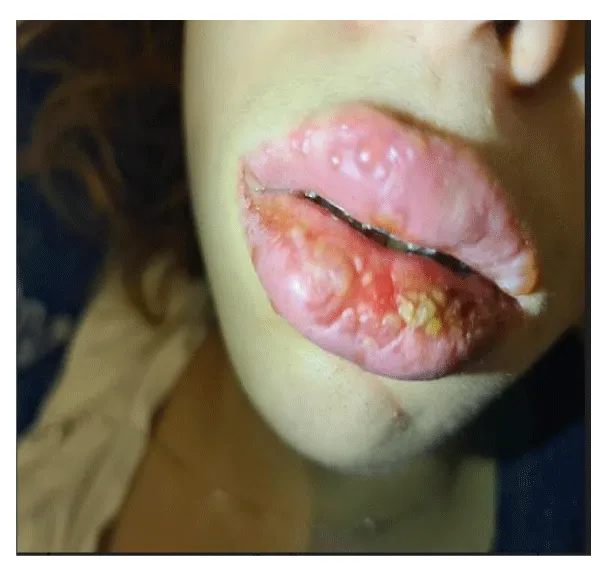
The condition did not improve so it was decided to combine 500 mg of valproate and lamotrigine in an increasing dose of half a 50 mg tablet for 1 week, then half a tablet every 12 hours for a week, and then 1 tablet every 12 hours. 17 days after starting this therapy, the patient presented small blisters on the upper lip (Figure 1)that within 24 hours spread to the entire interior of the mouth [4,5], extending to the vagina with the appearance of punctate lesions on the trunk (thorax and abdomen).
At the time of the onset of SJS, there were no risk factors such as HIV, cancer, or weakened immune system, a genetic study was performed to assess the risk factors, Genetic studies are not done in the country for Steven Johnson.
We believe that early recognition of symptoms contributes to effective therapeutic efficacy.
No novel therapy was performed on the patient.
The patient gave her consent for her case to be published, with some restrictions that appear in the annexes.
In the country, it does not exist Institutional Review Board (IBR).
Diagnosis
Stevens-Johnson Syndrome
Treatment:
The patient is admitted
Lamotrigine is discontinued
Physiological serum hydration 2 ml/kg/
Gentle cleansing of the skin with sterile water and intravaginal corticosteroid ointment
methylprednisolone 500 mg per day for three days intravenous immunoglobulin (IVIG; administered at a dose of 1.5 g/kg for five days The response to treatment was good, with no evidence of cutaneous, mucosal, or visceral symptoms six months after the condition (Figure 2), however, the patient’s fear of ingesting medications of any kind, persists (psychological sequelae) [6].
Pathogenesis
SJS is predominantly a drug-specific T cell-mediated reaction [7-10]. Human leukocyte antigen (HLA)-drug-T cell receptor (TCR) engagement results in activation of drug-specific CD8+ T cells with subsequent release of cytotoxic proteins, resulting in epidermal injury (necrosis).
The main pathogenic sequence can be summarized as follows:
- Genetic predisposition (HLA polymorphism and pharmacogenetics)
- Presentation of the drug antigen.
- T cell-mediated response and immune dysregulation.
- Release of cytotoxic mediators, death signals, and keratinocyte cell death.
Causal mechanisms of Stevens-Johnson syndrome due to lamotrigine
The mechanism of action of lamotrigine in inducing SJS is under debate. However, it has been proposed that, since its metabolism is mainly hepatic through glucuronidation by UGT1A4 and UGT2B7, certain genetic variations in these enzymes would compromise the clearance of the drug, increasing its serum concentrations [11]. The same happens with the concomitant use of valproic acid since it interferes with the metabolism of lamotrigine by inhibiting the glucuronide [12-17]. Likewise, it is suggested that rapidly increasing lamotrigine doses increase the risk of skin reaction. Other theories that have been postulated include that adverse reactions to anticonvulsants are secondary to the drug binding to the clone-specific T cell receptor, as drug-specific T cells have been identified for lamotrigine and carbamazepine.
In conclusion, it could be said that analyzing the main hypotheses about the probable pathophysiology of SJS, that there are two major pathways involved: the metabolic and the immune; The latter is the largest and best founded. Therefore, based on the study of this pathway, and without being certain, we consider that the participation of the immune system is essential as a trigger for the exaggerated response to the drug, either through direct recognition by T cells or due to the formation of haptens from the drug.
Clinical presentation
The disease begins with nonspecific symptoms such as fever and malaise, and upper respiratory tract symptoms such as cough, rhinitis, eye pain, and myalgia. Over the next three to four days, a rash with blisters and erosions appears on the face, trunk, extremities, and mucosal surfaces.
- Erythematous, target, annular, or purpuric macules.
- Flaccid blisters.
- Large painful erosions.
- Nikolsky positive (lateral pressure on the skin causes detachment of the epidermis).
Stevens-Johnson syndrome is characterized more by a target rash, with fewer areas of denudation.
Ulcerations and erosions of the mucosa can affect the lips, mouth, pharynx, esophagus and gastrointestinal tract, eyes, genitals, and upper respiratory tract. Approximately half of patients have involvement of three mucosal sites. The liver, kidneys, lungs, bone marrow, and joints may be affected.
Diagnosis
The diagnosis of SJS is based on clinical presentation, history, and supporting histologic evidence.
The diagnosis of a serious cutaneous adverse reaction should be suspected in any patient who presents with a sudden onset of a painful mucocutaneous rash associated with systemic symptoms and a history of exposure to the suspected drug for a prolonged period of one to four weeks (less commonly eight weeks) before the start of the reaction (Table 1).
Skin biopsy: Skin histology reveals keratinocyte necrosis, epidermal (or epithelial) necrosis, and mild lymphocytic dermal infiltration. Direct immune fluorescence is negative.
Laboratory studies: Complete blood count with differential, coagulation studies, metabolic panel (i.e., glucose, electrolytes, blood urea nitrogen, creatinine, calcium, total protein, albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase), sedimentation rate globular and C-reactive protein.
Bacterial and fungal cultures should be performed from blood, wounds, and mucosal lesions. Due to the high risk of bacterial superinfection and sepsis, cultures should be repeated at frequent intervals during the acute phase of the disease [17-22].
Procalcitonin may be useful as an early diagnostic marker of bacteremia. The presence of elevated procalcitonin and hypothermia has been associated with a higher incidence of positive blood cultures. Skin cultures have good negative predictive values for S. aureus and P. aeruginosa bacteremia if skin swabs are negative for these organisms.
A chest x-ray should be obtained in all patients due to the possibility of pulmonary involvement from SJS, pneumonia, and interstitial pneumonitis.
Prognosis
The severity of Stevens-Johnson syndrome is assessed using SCORTEN (Table 2). One point is earned for each of the following seven criteria upon admission.
- Age over 40 years
- Presence of a malignancy
- Heart rate of more than 120 bpm
- Initial percentage of epidermal detachment greater than 10%
- Serum urea level greater than 10 mmol/L
- Serum glucose level greater than 14 mmol/L
- Serum bicarbonate level less than 20 mmol/L
The risk of dying from Stevens-Johnson syndrome depends on the score.
The mortality rate is more than 40 times higher in those with bicarbonate levels less than 20 mmol/L compared to those with higher levels.
Treatment
Patients must undergo an interprofessional evaluation in a specialized hospital setting, Clinician, Intensivist, Dermatologist, Allergist, Plastic Surgery or Burn Specialist, Ophthalmologist, Gynecologist, Urologist, Respiratory Physician, Physiotherapist, and Nutritionist [23].
Caring for a patient with Stevens-Johnson syndrome requires:
- Stopping the suspected offending medication.
- Hospital admission: preferably in the intensive care and/or burn unit.
- Fluid replacement (crystalloid).
- Nutritional evaluation: may require nasogastric tube feeding.
- Temperature control: warm environment, emergency blanket.
- Pain relief.
- Supplemental oxygen and, in some cases, intubation with mechanical ventilation.
- Sterile/aseptic handling.
- Skin care requires daily examination of the skin and mucosal surfaces to detect infections, and non-adherent dressings, and avoid skin trauma. Mucosal surfaces require careful cleaning and topical anesthetics.
- Gentle removal of necrotic skin/mucous tissue.
- Culture of skin lesions, armpits, and groin every two days.
- Antibiotics may be required for secondary infection, but are best avoided prophylactically.
It is unknown whether systemic corticosteroids are beneficial, but they are often prescribed in high doses during the first three to five days of admission [24-27]. Granulocyte colony-stimulating factor (G-CSF) may be beneficial in patients with severe neutropenia.
Other drugs considered effective include systemic corticosteroids, cyclosporine, TNF-alpha inhibitors [17], N-acetylcysteine, and intravenous immunoglobulins.
Conclusion
The management of SJS is interprofessional. The acute care of these patients is provided by wound care. The doctor must closely evaluate the medications the patient ingests. In this case, avoid carbamazepine, which has a probable cross-reaction. May require mental health counseling. Following discharge, the patients need long-term follow-up to ensure that there are no functional déficits. Once a patient has suffered an SJS, it is highly recommended that the patient wear a warning bracelet indicating the toxic agent or allergen.
- World Health Organization. International drug monitoring: the role of national centres. Geneva, Switzerland: World Health Organization; 1972. Available from: https://www.who-umc.org/media/2680/who-technical-report-498.pdf
- Wong S, Tham M, Goh C, Cheong H, Chan S. Spontaneous cutaneous adverse drug reaction reports—an analysis of a 10-year dataset in Singapore. Pharmacol Res Perspect. 2019;7(2). Available from: https://doi.org/10.1002%2Fprp2.469
- Mustafa S, Ostrov D, Yerly D. Severe cutaneous adverse drug reactions: presentation, risk factors, and management. Curr Allergy Asthma Rep. 2018;18:26. Available from: https://doi.org/10.1007/s11882-018-0778-6
- Rodríguez-Martín S, Martín-Merino E, Lerma V, Rodríguez-Miguel A, González O, González-Herrada C, et al. Incidence of Stevens-Johnson syndrome/toxic epidermal necrolysis among new users of different individual drugs in a European population: a case-population study. Eur J Clin Pharmacol. 2019;75:237-246. Available from: https://doi.org/10.1007/s00228-018-2569-3
- Alyahya B, Friesen M, Nauche B, Laliberté M. Acute lamotrigine overdose: a systematic review of published adult and pediatric cases. Clin Toxicol. 2018;56(2):81-89. Available from: https://doi.org/10.1080/15563650.2017.1370096
- Mullan KA, Anderson A, Illing PT, Kwan P, Purcell AW, Mifsud NA. HLA-associated antiepileptic drug-induced cutaneous adverse reactions. HLA. 2019;93(6):417-435. Available from: https://doi.org/10.1111/tan.13530
- Parveen S, Javed MA. Stevens-Johnson syndrome associated with lamotrigine. Pak J Med Sci. 2013;29(6):1450-1452. Available from: https://doi.org/10.12669%2Fpjms.296.4385
- Velázquez-Cárcamo EA, Rodríguez-Chávez Y, Méndez-Flores S, Domínguez-Cherit J. Lamotrigine and its relationship with Stevens-Johnson syndrome and toxic epidermal necrolysis. Rev Med Inst Mex Seguro Soc. 2020;58(2):202-205. Available from: https://doi.org/10.24875/RMIMSS.M20000018
- Chaby G, Maldini C, Haddad C, Lebrun-Vignes B, Hemery F, Ingen-Housz-Oro S, et al. Incidence and mortality from epidermal necrolysis (Stevens-Johnson syndrome/toxic epidermal necrolysis) in France during 2003-16: a capture-recapture estimate from four sources. Br J Dermatol. 2020;182:618. Available from: https://doi.org/10.1111/bjd.18424
- Zhu J, Chen G, He Z, Zheng Y, Gao S, Li J, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: a safety analysis of clinical trials and an FDA pharmacovigilance database. EClin Med. 2021;37:100951. Available from: https://doi.org/10.1016%2Fj.eclinm.2021.100951
- Liew YCC, Choo KJL, Oh CC, Pang SM, Yeo YW, Lee HY. Stevens-Johnson syndrome/toxic epidermal necrolysis induced by Mycoplasma: a case-control analysis from a cohort managed at a specialized center. J Am Acad Dermatol. 2022;86:811. Available from: https://doi.org/10.1016/j.jaad.2021.04.066
- Zou H, Daveluy S. Toxic epidermal necrolysis and Stevens-Johnson syndrome after COVID-19 infection and vaccination. Australas J Dermatol. 2023;64(1):e1-e10. Available from: https://doi.org/10.1111/ajd.13958
- Calley BJ, Saleh J, Young K, Wanat KA. Stevens-Johnson syndrome in a pregnant woman who received the influenza vaccine. JAAD Case Rep. 2022;23:35. Available from: https://doi.org/10.1016%2Fj.jdcr.2022.02.002
- Gibson A, Deshpande P, Campbell CN, Krantz MS, Mukherjee E, Mockenhaupt M, et al. Updates on immunopathology and genomics of severe cutaneous adverse drug reactions. J Allergy Clin Immunol. 2023; 151(2):289-300.e4. Available from: https://doi.org/10.1016/j.jaci.2022.12.005
- Koh HK, Fook-Chong S, Lee HY. Evaluation and comparison of ABCD-10 and SCORTEN performance in predicting toxic epidermal necrolysis outcomes. JAMA Dermatol. 2020;156(12):1294-1299. Available from: https://jamanetwork.com/journals/jamadermatology/fullarticle/2771824
- Duplisea MJ, Roberson ML, Chrisco L, Strassle PD, Williams FN, Ziemer CM, et al. Performance of the ABCD-10 and SCORTEN mortality prediction models in a cohort of patients with Stevens-Johnson syndrome/toxic epidermal necrolysis. J Am Acad Dermatol. 2021; 85(4):873-877. Available from: https://doi.org/10.1016/j.jaad.2021.04.082
- Crowder CA, Jeney SES, Kraus CN, Bernal N, Lane F. Vulvovaginal involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis: management and techniques used to reduce gynecological sequelae. Int J Dermatol. 2022; 61(2):158-163. Available from: https://doi.org/10.1111/ijd.15676
- Mieno H, Ueta M, Kinoshita F, Teramukai S, Kinoshita S, Sotozono C. Pulse corticosteroid therapy for patients with Stevens-Johnson syndrome and toxic epidermal necrolysis with acute ocular involvement. Am J Ophthalmol. 2021; 231:194-199. Available from: https://doi.org/10.1016/j.ajo.2021.06.015
- Hall LN, Shanbhag SS, Rashad R, Chodosh J, Saeed HN. Systemic cyclosporine effects on acute Stevens-Johnson syndrome/toxic epidermal necrolysis ocular disease. Ocul Surf. 2021;19:128-132. Available from: https://doi.org/10.1016/j.jtos.2020.05.003
- Zhang J, Lu CW, Chen CB, Wang CW, Chen WT, Cheng B, et al. Evaluation of combined etanercept and systemic corticosteroids therapy for Stevens-Johnson syndrome and toxic epidermal necrolysis: a multicenter observational study. J Allergy Clin Immunol Pract. 2022;10(5):1295-1304.e6. Available from: https://doi.org/10.1016/j.jaip.2022.01.038
- Ao S, Gao X, Zhan J, Ai L, Li M, Su H, et al. Tumor necrosis factor-alpha inhibition enhances conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a patient cohort. J Am Acad Dermatol. 2022; 86(6):1236-1245. Available from: https://doi.org/10.1016/j.jaad.2022.01.039
- Safiri S, Ashrafi-Asgarabad A. The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs: commentary on data scarcity. Epilepsia. May 2018;59(5):1083-1084. Available from: https://doi.org/10.1111/epi.14024
- Allende Bandrés MA, Izuel Rami M, Urbieta Sanz E, Villar Fernández I, Carcelén Andrés J. Cross-sensitivity syndrome among antiepileptics: a case report. Farm Hosp. 2004;28(1):56-58. Available from: https://pubmed.ncbi.nlm.nih.gov/15012179/
- Mullins L, Guajardo M, Fuenzalida M, Clavero Ch. Adverse cutaneous reactions to anticonvulsants and mood stabilizers. Rev Chil Dermatol. 2011;27(1):71-76.
- Carias C, Paredes Y. Stevens-Johnson syndrome associated with lamotrigine. Rev Hond Post Psiq. 2013;27.
- Andreoli MX, Tellez M, Guglielmone A, Velázquez CM, Dilsizan VN. Progression to toxic epidermal necrolysis due to lamotrigine use: a case report. Rev Argent Dermatol. 2008;89(3):188-92.
- Fernández-Calvo C, Olascoaga J, Resano A, Urcola Echeverría J, Turneu A, Zubizarreta J. Lyell syndrome associated with lamotrigine. Rev Neurol. 2000;31:1162-1164. Available from: https://pubmed.ncbi.nlm.nih.gov/11205551/
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
