Journal of Novel Physiotherapy and Physical Rehabilitation
Right Ventricular Dysfunction is related with Poor Exercise Tolerance in Elderly Patients with Heart Failure with Preserved Ejection Fraction
Chiara Fossati1,2, Valentino D’Antoni1, Jeganath Murugesan3, Deborah Fortuna1, Serena Selli1, Noemi Punzo1, Giuseppe Caminiti1*
2Department of Movement, Human and Health Sciences, Università degli Studi di Roma “Foro Italico”, Piazza Lauro de Bosis 15, 00194 Rome, Italy
3Dipartimento di Medicina Fisica e Riabilitazione, Università Tor Vergata, Rome, Italy
Cite this as
Fossati C, D’Antoni V, Murugesan J, Fortuna D, Selli S, et al. (2017) Right Ventricular Dysfunction is related with Poor Exercise Tolerance in Elderly Patients with Heart Failure with Preserved Ejection Fraction. J Nov Physiother Phys Rehabil 4(1): 021-026. DOI: 10.17352/2455-5487.000041Background: Exercise intolerance (EI) is a cardinal feature in subjects with heart failure with preserved ejection fraction (HFpEF). Factors related to EI in such patients are not completely understood. The association between right ventricular (RV) dysfunction and pulmonary hypertension (PH) with EI has been poorly investigated so far. We hypothesized that RV function measured by Tricuspid Annular Plane Systolic Excursion (TAPSE)/Pulmonary Arterial Systolic Pressure (PASP) ratio would predict EI assessed by 6-Minute Walking test (6MWT) in elderly patients with HFpEF
Methods: This was a retrospective study in which data of one hundred twenty-six patients with HF and left ventricular ejection fraction >50%, were collected. All subjects underwent an echocardiographic evaluation and a 6MWT.
Results: Overall 43 out of 126 (34.1%) patients had RV dysfunction according to TAPSE value. TAPSE (r=0.28 p=0.042) and PASP (r=-0.33 p=0.028) were both significantly related to 6MWT distance. In a multivariate regression analysis PASP (adjusted OR = 1.39; 95% CI=1.18–1.66, p= 0.044) and TAPSE (adjusted OR = 1.43; 95% CI=1.22–2.65, p= 0.013) resulted independently related to EI. The median value of TAPSE/PASP ratio was 0.46. TAPSE/PASP ratio was significantly related to the 6MWT distance (r=0.48; p=0.007). Patients with TAPSE/PASP ratio<0.46 had larger RV diastolic diameter, higher NYHA class and were more likely to have atrial fibrillation and be treated with diuretics.
Conclusions: RV dysfunction, measured by TAPSE/PASP ratio, correlates strongly with EI in HFpEF patients and identify a subgroup of patients with worse clinical conditions. We recommend the routine assessment of RV function in HFpEF, in order to better characterize patients risk profile and to plan their rehabilitative program.
Introduction
Exercise intolerance (EI) is a cardinal feature in subjects with heart failure with both reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF). In such patients EI is a multifactorial condition generated by peripheral and central derangements that occur during the disease progression [1,2]. Despite several factors related to EI in HFpEF have been recently elucidated [3,4] there is still a need for further investigations on this topic.
PH and RV dysfunction and pulmonary hypertension (PH), have a well-established correlation with EI in HFrEF [5-7]. PH and RV dysfunction are common and tightly connected in patients with HFpEF [8] and powerful predictors of poor outcome in those patients [9,10], the relationship between RV dysfunction and EI in these patients has been poorly investigated until now.
RV function assessment is a difficult task because it is influenced by several parameters including the chamber’s shape, presence of a separate infundibulum, prominent trabeculations, and dependence on loading conditions [11,12]. Several echocardiographic indices of RV function have been developed but no one of them likely adequately quantify the complexity of RV contractility; therefore there is not a general agreement on which is the most reliable [13]. Tricuspid Annular Plane Systolic Excursion (TAPSE) estimates longitudinal basal to apical motion of the RV free wall. Despite it is simply a surrogate marker of longitudinal RV systolic function and does not reflect the contractile state of the RV, the assessment of TAPSE seems particularly useful in clinical practice providing a simple and rapid quantitative method for evaluating RV performance in patients with HF [14].
Despite the direct measurement of pulmonary pressure represents the gold standard for the assessment of RV afterload, Doppler estimation of Pulmonary Arterial Systolic Pressure (PASP) is feasible, often measurable, and clinically relevant in patients with HF [15-17]. PASP can be used as a surrogate to diagnose the presence and severity of PH.
The ratio between TAPSE and PASP is an index which has been recently introduced by Italian researchers as surrogate of RV function. In the author intentions the combination of TAPSE and PASP, by reproducing in-vivo the length-to-force relationship for the right ventricle, overcomes, at least partially, the limitations of using TAPSE alone, and permits a more comprehensive evaluation of RV function. TAPSE/ PASP ratio showed a significant prognostic power in patients with HF, independently from the left ventricular ejection fraction that was stronger than that provided by TAPSE. [18]. Moreover TAPSE/ PASP ratio can be easily obtained by physicians in different clinical settings including cardiac rehabilitation.
In this study we hypothesized that a low TAPSE/PASP ratio would be related with EI, measured by 6-Minute Walking Test (6MWT) distance, in a large population of elderly patients with HFpEF.
Methods
Study population
The study was performed at Cardiac Rehabilitation Department of IRCCS San Raffaele Pisana, Rome, Italy between January 2011 and September 2014. We collected data of one hundred twenty six patients with HFpEF, over 70 years old (mean age: 77.9±14 years; male/female = 84/42, NYHA functional class II/III = 85/41. Patients were diagnosed as HFPEF in accordance with current guidelines [19]. Subjects with significant valvular heart disease (moderate to severe stenosis/regurgitation), hypertrophic or infiltrative cardiomyopathy, pericardial diseases, unstable ischemic heart disease (acute coronary syndrome or recent myocardial infarction<60 days before selection) were excluded. Other exclusion criteria were: inability to perform 6MWT and/or poor acoustic window. Patients were defined as diabetic if they had fasting glycemia >124 mg/dl and/or were treated with antidiabetic drugs; patients were considered having chronic obstructive pulmonary disease (COPD) if they had a well-established diagnosis of the disease with available spirometry results and previous hospitalizations for worsening respiratory function and/or if they were currently treated with steroids or bronchodilator agents. Subjects were categorized as having coronary artery disease (CAD) if they had previous coronary acute events and or coronary interventions.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was a priori approved by the internal Ethics Committee. All subjects provided written consent for inclusion in this study. Data of those subjects who met the inclusion/exclusion criteria were collected. All patients underwent an echocardiographic evaluation and performed a 6MWT on the same day before starting a rehabilitative program.
Echocardiographic evaluation
A comprehensive two-dimensional echocardiographic study with conventional Doppler and tissue Doppler was performed using an Acuson Sequoia device and a 2.5 to 4.25-MHz wide-angle phased-array transducer. Using the apical four-chamber view, an M-mode cursor was placed through the lateral tricuspid annulus in real time to obtain TAPSE. It was measured as the peak excursion of the tricuspid annulus (millimeters) from the end of diastole to end systole, with values representing TAPSE being averaged over three to five beats. Tissue Doppler signals were recorded in all patients. RV systolic pressure was determined from the tricuspid regurgitation (TR) jet velocity using the simplified Bernoulli equation, and combining this value with an estimate of the right atrial pressure obtained by the diameter and collapsibility of the inferior vena cava that was added to the calculated gradient to yield PASP. Atria measurement were done from the apical four-chamber view at end-systole. Left atrial enlargement was defined as left atrial area > 20 cm2. Right atrial enlargement was defined as right atrial minor axis >45mm [20]. Longitudinal RV systolic dysfunction was defined categorically as TAPSE<16mm [13].
Assessment of exercise tolerance
Exercise tolerance was evaluated by 6MWT that was carried out after echocardiographic evaluation. The test was performed according to the standardized procedure [21] and was supervised by a physical therapist. Patients were asked to walk at their own maximal pace a 100m long hospital corridor with 10-meter signs on the floor. Every minute a standard phrase of encouragement was told. Patients were allowed to stop if signs or symptoms of significant distress occurred (dyspnea, angina), though they were instructed to resume walking as soon as possible. Results of 6MWT were expressed in distance walked (meters).
Statistical analysis
Results were expressed as median ± standard deviation (SD) or percentages were appropriate. Chi-square tests for trend and unpaired t-test were used to compare differences of each parameter between different groups of patients divided according to the values of TAPSE and PASP and TAPSE/PASP ratio. Bivariate linear regression (Pearson) analysis was performed to examine the relationship between TAPSE/PASP ratio and 6MWT distance. A multivariate regression analysis was performed to determine the independent relation between TAPSE and PASP and 6MWT distance. The model included, several available covariates, affecting functional measure. The population was divided into two groups according with the median value of TAPSE/PASP ratio (0.46). A p value < 0.05 was considered statistically significant for all tests.
Results
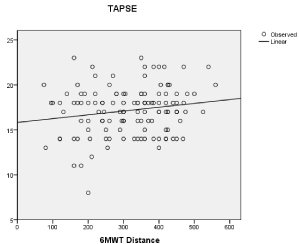
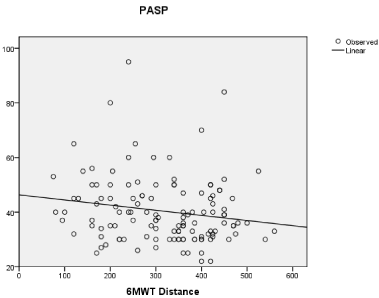
Participants were mainly hypertensive (77.6%) and men (66.6%). Overall 43 out of 126 (34.1%) patients had longitudinal RV systolic dysfunction according to TAPSE value. Subjects with TAPSE<16 had higher value of PASP compared to those with a TAPSE≥16 (56.8±18 vs 33.4±14; p 0.002). The mean value of 6MWT distance was significantly lower for patients with TAPSE<16 compared to those with TAPSE ≥16 (268.6±88 vs 346.3±67, p 0.0001). When evaluated as continuous variables, PASP(r=-0.33 p=0,028) and TAPSE (r=0.28 p=0,042) were both significantly related to 6MWT distance (Figure 1 A,B).
The independent correlation of TAPSE and PASP with ET in our sample was evaluated through a multivariate regression analysis in which we included the following covariates: age, gender, non-paroxysmal atrial fibrillation, diabetes, hemoglobin, left ventricular ejection fraction, left ventricular diastolic function, and renal failure. After adjusting for these covariates TAPSE and PASP resulted both independently related to EI in our population (TAPSE adjusted OR=1.43; 95% CI=1.22–1.65, p=0.013; PASP adjusted OR = 1.39; 95% CI=1.18–1.66, p= 0.044).
The median value of TAPSE/PASP ratio in our population was 0.46. Table 1 shows the clinical characteristics of the patients stratified by TAPSE/PASP ratio. Subjects with TAPSE/PASP ratio<0.46 had significantly lower values of TAPSE and higher values of PASP compared to those with ratio ≥ 0.46 as showed in Table 2. They had also a worse NYHA functional class (Table 1). The two groups had similar diastolic function, left ventricular ejection fraction, resting heart rate and blood pressure (Tables 1,2). Patients with TAPSE/PASP ratio<0.46 walked a significantly shorter distance at 6MWT, had larger RV diastolic diameter and were more likely to have atrial fibrillation and be treated with diuretics than patients with TAPSE/PAPS ratio≥ 0.46 (Table 2).
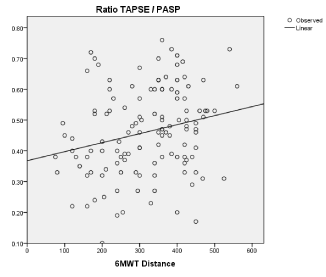
When considered as continuous variable, TAPSE/PASP ratio was strongly related to 6MWT distance (r=0.46; p 0.007)(Figure 2).
Discussion
In this study TAPSE/PASP ratio, a new index of RV function, was independently and positively related to 6MWT distance in a large population of elderly patients with HFpEF. Other studies evaluating the relation between RV function and EI, in different populations and with different modalities, have been recently published [26,27]. Our study presents some original features. Firstly it confirmed the existence of a correlation between RV function and EI, previously documented by these studies in subjects with CAD and COPD, by using a new index never tested specifically in HFpEF subjects before. Secondly for the first time it extended this correlation to a population of elderly patients with HFpEF.
EI is a cardinal feature of HFpEF and similarly to patients with HFrEF it correlates with reduced quality of life and higher mortality rate [24-26]. Pathophysiological mechanisms underlying EI in HFPEF are still not entirely known. Reduced chronotropic reserve [27], reduced left ventricular inotropic reserve [28] and skeletal muscle abnormalities [5] have been described as determinant of EI in HFpEF. Our data indicate that the deterioration of RV function observed during HF progression might contribute to the onset of EI in patients with HFpEF. In our opinion, though our results need further confirmatory studies, they suggest that EI in HFpEF patients could be counteract by interventions aimed to reduce pulmonary pressure and/or to directly improve RV function.
Interestingly, at the multivariate regression analysis, we observed that TAPSE and PASP were both independent predictors of 6MWT distance. This could be considered an unexpected result because TAPSE and PASP are strongly linked from a pathophysiological point of view: in HF, an increase in RV afterload through the development of PH secondary to increased left-sided filling pressures promotes RV remodeling and failure. A possible explanation of this result is that though in our population the main factor responsible for RV dysfunction was an abnormal increase of afterload, other mechanisms causing a primitive damage of RV myocardium were certainly involved. This hypothesis is strengthened by the observation that an underlying coronary artery disease was present in almost 60% of patients and about 35% of them had history of AMI.
In this study, exercise tolerance was assessed by the 6MWT distance, a measure of submaximal exercise capacity. We had no data on maximal oxygen consumption of our patients because VO2 peak is a parameter barely achievable in elderly subjects. Conversely, 6MWT distance is easier to get and better reflects the capacity to perform the everyday life activities in such patients [29].
Interestingly the median value of TAPSE/PASP ratio in our study was higher compared to the value used in the study of Guazzi et al [18]. In our opinion, this discrepancy likely reflects different inclusion criteria used by the two studies: Guazzi et al. included patients with both HFrEF and HFpEF while we included only HFpEF subjects. Therefore, our data suggest that patients with HFpEF have a milder degree of RV dysfunction compared with HFrEF. A similar result has been observed by Puwanant et al. [30], who evaluated a population of heart failure patients with both reduced and preserved ejection fraction and demonstrated that the severity of RV dysfunction increased with increasing severity of left ventricular dysfunction.
In our population, a TAPSE/PASP ratio <0.46 was associated with more severe symptoms and greater comorbidity burden. In particular, these patients had a worse NYHA class, an higher rate of non-paroxysmal atrial fibrillation, a greater occurrence of left atrial and right ventricle enlargement and were more often using diuretics. This result appears to be consistent with other studies in which the prognostic implications of RV dysfunction and/or PH in HFpEF have been evaluated. In a recent community based study, Mohammed et al. [10], showed that RV dysfunction, assessed by TAPSE, was associated with a worse clinical and echocardiographic profile and with a poorer outcome.
In our population subjects with TAPSE/PASP ratio <0.46 had similar left ventricular ejection fraction and left ventricular diastolic function compared to those with TAPSE/PASP ratio ≥0.46. This suggest that the worse RV function in the group with TAPSE/PASP ratio <0.46 could explain such clinical differences. Therefore, the evaluation of TAPSE/PASP ratio in HFpEF could help physicians to identify patients with greater functional limitation and worst prognostic profile. In this study the prevalence of longitudinal RV systolic dysfunction, defined as TAPSE < 16 mm, was 34.1%. The assessment of RV function by echocardiography remains very challenging and its prevalence varies considerably depending on which kind of measure is used [30,31]. In a small observational cohort with HFpEF, 28% of patients had a TAPSE <16 mm, and 14% had a RV fractional area change <35% [32]. However, our data are consistent with those of a study in which RV dysfunction was assessed by right heart catheterization in which RV dysfunction was present in 33% of patients with HFpEF [9].
Study limitations
In this retrospective study we included all subjects with diagnosis of HFpEF who met eligibility criteria. Despite the protocol did not include “a priori” age-related restrictions we enrolled only elderly patients and this can limit our conclusions. Given the advanced age and high rate of comorbidities of our patients their exercise tolerance was evaluated only through the walking test distance and we have not data on their maximal exercise capacity. We ruled out subjects with poor acoustic windows such as those with severe obesity or advanced COPD. Therefore, our results may not be applicable to the entire HFpEF population. Another important limitation is the lack of invasive right heart hemodynamic and pulmonary pressure assessment. We estimated RV dysfunction by the use of only one method; however, the prevalence of RV dysfunction depends on the method used for assessing it and other authors found an higher prevalence of RV dysfunction using TAPSE compared to other methods [30,31]; hence we cannot exclude an overestimation of RV dysfunction among our patients. RV diastolic function was not assessed in this population.
Conclusions
We observed that RV dysfunction affects EI in elderly patients with HFpEF and reflects a worse clinical profile of these patients. Together with other recent studies, our data strengthen the idea that RV function has a fundamental role on determining clinical conditions and prognostic profile of HFpEF patients. We think that our study helps to clarify which factors contribute to EI in HFpEF by identifying RV dysfunction as a possible determinant of EI in such patients. We believe that the mechanisms underlying this correlation cannot be explained by this study, because of its retrospective design, and should be investigated in properly designed prospective studies. In light of the results of this study we recommend the routine assessment of RV function by echocardiography in order to better characterize the risk profile of patients with HFpEF.
- Clark AL (1997) The origin of symptoms in chronic heart failure. Heart 78: 429-443. Link: https://goo.gl/6BUbor
- Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corrà U, et al. (2010) Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehab. 17: 637–642. Link: https://goo.gl/dt0HW2
- Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, et al., (2011) Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 58: 265-274. Link: https://goo.gl/mk74DO
- Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, et al. (2013) Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 15: 776–785. Link: https://goo.gl/2WeCvL
- Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, et al. (2015) Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail;8: 286-294 Link: https://goo.gl/VZML0m
- Butler J, Chomsky DB, Wilson JR (1999) Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 34: 1802-1806. Link: https://goo.gl/lppmBf
- Caminiti G, Volterrani M, Murugesan J, Baratta P, D'Antoni V, et al. (2013) Tricuspid annular plane systolic excursion is related to performance at six minute walking test in patients with heart failure undergoing exercise training. Int J Cardiol. 169: 91-92. Link: https://goo.gl/HdAryh
- Guazzi M (2014) Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail 7: 367-377. Link: https://goo.gl/20rcG0
- Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA (2013) Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 35: 3452-3462. Link: https://goo.gl/qEctXQ
- Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, et al. (2014) Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 130: 2310-2320. Link: https://goo.gl/2WyGvA
- Barnard D, Alpert JS (1987) Right ventricular function in health and disease. Curr Probl Cardiol. 12: 417–449. Link: https://goo.gl/DX6xhO
- Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, et al., (1985) An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 5: 918–927. Link: https://goo.gl/HUi5Kd
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, et al. (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J of the Am Soc Echocardiogr. 23: 685-713. Link: https://goo.gl/Xiny7f
- Meluzín J, Spinarová L, Bakala J, Toman J, Krejcí J, et al. (2001) Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 22: 340–348. Link: https://goo.gl/lmOXcn
- Ghio S, Temporelli PL, Klersy C, Simioniuc A, Girardi B, et al. (2013) Prognostic relevance of a non-invasive evaluation of right ventricular function and pulmonary artery pressure in patients with chronic heart failure. Eur J Heart Fail. 15: 408-414. Link: https://goo.gl/DZO2Vs
- Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, et al. (2000) Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 85: 837–842. Link: https://goo.gl/5iuVyG
- Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, et al. (2012) Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 59: 222–231. Link: https://goo.gl/oGTiq6
- Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, et al., (2013) Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 305: H1373-H1381. Link: https://goo.gl/kaAd78
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. (2016) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 37: 2129–2200. Link: https://goo.gl/rYTZcx
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.. J Am Soc Echocardiogr 18: 1440-1463. Link: https://goo.gl/6C00rx
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six minute walk test. Am J Respir Crit Care Med 166: 111–117. Link: https://goo.gl/mLDCHG
- Kim J, Di Franco A, Seoane T, Srinivasan A, Kampaktsis PN, et al. (2016) Right Ventricular Dysfunction Impairs Effort Tolerance Independent of Left Ventricular Function Among Patients Undergoing Exercise Stress Myocardial Perfusion Imaging. Circ Cardiovasc Imaging 2016; 9: e005115. Link: https://goo.gl/kvufG4
- Caminiti G, Cardaci V, Conti V, DʼAntoni V, Murugesan J, et al., (2015) Right ventricular systolic dysfunction is related to exercise intolerance in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev.35: 70–74. Link: https://goo.gl/M4cp1P
- Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, et al. (2002) Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA;288: 2144–2150. Link: https://goo.gl/w72hBV
- Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW (2015) Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol 12: 294–304. Link: https://goo.gl/mEF8Gg
- Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ (1991) Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 17: 1065–1072. Link: https://goo.gl/1vjYoF
- Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, et al.. (2006) Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 114: 2138–2147. Link: https://goo.gl/Ql3bes
- Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM (2010) Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 56: 855–863. Link: https://goo.gl/GgT3Sf
- Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG (2013) Clinically meaningful change estimates for the Six-Minute Walk Test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J 24: 21–29. Link: https://goo.gl/FVHEjm
- Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, et al. (2009) Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr 10: 733–737. Link: https://goo.gl/DVbEBa
- Morris DA, Gailani M, Vaz Pérez A, Blaschke F, Dietz R, et al. (2011) Right ventricular myocardial systolic and diastolic dysfunction in heart failure with normal left ventricular ejection fraction. J Am Soc Echocardiogr 24: 886–897. Link: https://goo.gl/jc0VYS
- Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, et al. (2014) Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 72: 288–299. Link: https://goo.gl/SEZu0J
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
