Rheumatica Acta: Open Access
IgA Nephropathy Precedes the Onset of the Rheumatic Disease in a Female with Ankylosing Spondylitis
Haider M Al Attia1* and Mariano R Carballo2
2Department of Internal Medicine and Rheumatology and Radiology, Universal Hospital, Abu Dhabi, UAE
Cite this as
Al Attia HM, Carballo MR (2017) IgA Nephropathy Precedes the Onset of the Rheumatic Disease in a Female with Ankylosing Spondylitis. Rheumatica Acta: Open Access 1(1): 020-022. DOI: 10.17352/raoa.000005Case Report
IgA nephropathy (known as Berger’s disease) is the most common cause of glomerulonephritis. Its estimated prevalence between 25-50 cases per 100.000 individuals. Gross hematuria (40-50%) and microscopic hematuria (30-40%) are the most common findings which can be related to upper respiratory tract or gastrointestinal infection. Between 15-40% of affected individuals may progress to chronic renal failure [1-5]. The definitive diagnosis is made by renal biopsy findings which is characterized by mesangial IgA deposition. Although the condition is a limited non-systemic renal disease, some systemic diseases are sporadically associated with mesangial IgA deposition. Henoch-Schonlen purpura (HSP), systemic lupus erythematosus (SLE), hepatitis, dermatitis herpetiformis, celiac disease and ankylosing spondylitis (AS) have been closely linked to the IgA nephropathy [6,7].
The true prevalence of IgA nephropathy in AS is not yet known however, recent reports concluded a prevalence of hematuria (up to 20% ) of patients, whereas histopathologically confirmed IgA nephropathy is present in about 2% [8]. Although AS tends to occur predominantly in men (3-4:1), the previous reports surprisingly described IgA nephropathy as exclusively occurring in the male population of AS, and following the course of the established rheumatic disease. Only one report in the literature had described IgA nephropathy in a female with AS [9]. In this report, we describe another female in whom the onset of AS occurred after the renal manifestations of the IgA nephropathy.
A- 31 year old Indian and a mother of one child was referred by the orthopedic surgeon for further evaluation of a 1 year long problem of back and heel pain that has worsened recently. The pain was worse in the early hours of the morning. At the age of 29 she was managed by the nephrologist for episodic microscopic and gross hematuria, and subnephrotic proteinuria. The patient was neither hypertensive nor had a history of passing urinary stones, urinary tract infection, GI complaint, skin rash or a history suggestive of hepatitis or connective tissue disease (CTD). In addition to the hematological and biochemical work ups she underwent renal biopsy that furnished the diagnosis of IgA nephropathy. The immunofluorescent histology revealed eight glomeruli one of which was globally sclerotic, there was 3+ granular global mesangial positivity for IgA with negative IgG, trace IgM, negative C1q, C3 (3+), negative albumin, negative fibrinogen, trace kappa , and lambda(3+). She was commenced on ramipril since, with fairly reasonable response. The hematuria lessened significantly from 20-30 to 8-10/HPF and proteinuria from 956 to 785 mg /24 hours. At any point in time the renal function (serum urea and creatinine) remained within normal values. Likewise were the serum Ca, P, Na, K , CL, uric acid , albumin, glucose and the lipid profile. The MRI lumbar scan then showed focal right foraminal protrusion of L3-L4 and L4-L5 disc lesion, hemangioma in L2 vertebral body.
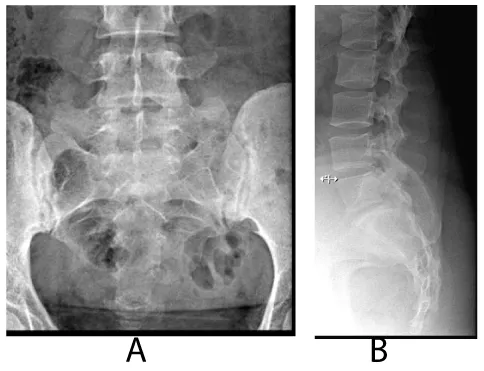
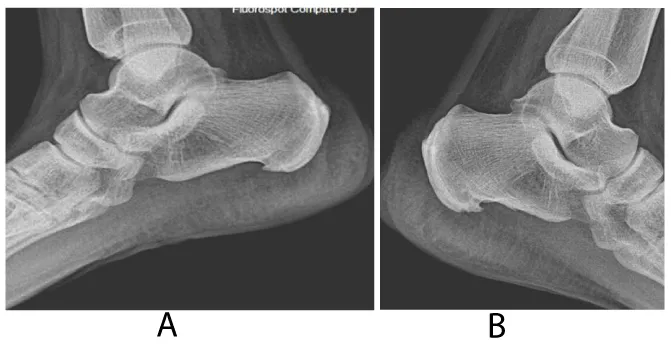
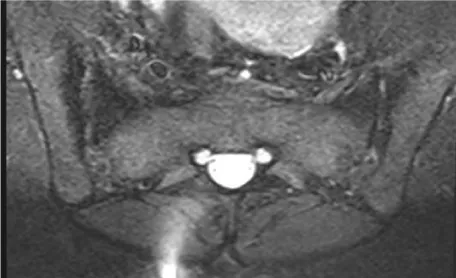
Our clinical evaluation revealed positive Faber‘s test along with positive schober’s test of 3 cm, chest expansion of 4 cm and tender heels. Other systems examination was unremarkable. Plain x-rays of the lumbosacral spine and sacroiliac joints (SI J) (Figure 1a,b demonstrates grade II-III sacroiliItis) along with loss of lumbar lordosis and bilateral calcaneal spurs (Figure 2). The MRI of SIJ was also consistent with sacroiliitis and sclerosis (Figure 3). The family history was negative for same condition. The latest biochemistry and hematology work ups were within normal values including CRP of 4.22 mg/dL (N <5) and ESR of 8 mm/HR. Serum ANF, Hepatitis B surface antigen, RF, cyclic citrullinated peptide (CCP) antibody were negative. Both of anti-ds–DNA antibody and serum complement components were normal. The serum IgA concentration was raised at 460 mg/dl (N 61-356 mg/dl). HLA B27 was negative.Reports on the concomitance between the IgA nephropathy and spondyloarthropathy (SpA) began to appear in the literature since the mid-seventies [10,11-13], indicating that individuals with SpA are more prone to develop I gA nephropathy than the general population. Its association with other seronegative spondyloarthropathies such as Behcet’s disease, psoriatic arthritis, Reiter’s syndrome and the postenteritic arthritides have been previously reported [5,6,10,12,13].
IgA nephropathy and AS are believed to share a common etiopathogenic mechanism. This mechanism involves the decreased expression of the receptor responsible for the clearance of IgA and its immune complexes on the surface of monocytes and neutrophils [12,14]. Interestingly, all the cases previously reported were of males and perhaps in accordance with the fact that the prevalence of AS in men is higher than that in women. Most were B27 positive [3,5,9,11,12,15]. The first case a female with AS/IgA nephropathy reported earlier was in fact a B27 positive patient [9].
Our patient with renal biopsy proven IgA nephropathy had clinical and radiological sacroiliitis and calcaneal spurs. She met the diagnosis of definite AS according to New York criteria [16]. This would make the patient as the second reported women with such concurrence in the English literature, yet of interest her renal features had manifested earlier than the usual rheumatic disease and differed from the previous one of being B27 negative.
This could suggest that there is a subset of AS patients in whom the concurrence of IgA nephropathy is not HLA- B27 associated or a male-gender specific. Rheumatologists and nephrologists’ alike need to be aware of the presence of such association in a subset of females with HLA B27 –negative AS.
- Berger J, Hinglais N (1968) Les depots intercapillaires d IgA-IgG. J Urol Nephol 74 : 694-695. Link: https://goo.gl/nfEOoG
- Galla JH (1995) IgA nephropathy. Kidney Int 47: 377-387. Link: https://goo.gl/dOXRx9
- Floege J, Feehally J (2000) IgA nephropathy: recent developments. J Am Soc Nephrol 11: 2395-2403. Link: https://goo.gl/kHz1cx
- D’Amico G (1987) The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709-727. Link: https://goo.gl/6wEgDO
- Power DA, Muirhead N, Simpson JG, Nicholls AJ, Horne CH, et al. (1985) IgA nephropathy is not a rare disease in the United Kingdom. Nephron 40: 180-184. Link: https://goo.gl/vZ1Gqk
- Hahn WH, Suh JS, Cho BS, Kim SD (2009) The enabled homolog gene polymorphisms are associated with susceptibility and progression of childhood IgA nephropathy. Exp Mol Med 41: 793-801. Link: https://goo.gl/RQyWxa
- IgA Nephropathy, Susceptibility to1, IgAN1; Online Mendelian inheritance in Man (OMM).
- Levy-Clarke G, Nussenblatt R (2006) Does anti-TNF therapy decrease the incidence of anterior uveitis in patients with ankylosing spondylitis? Nat Clin Pract Rheumatol 2: 72-73. Link: https://goo.gl/jgxvOG
- Lai KN, Li PKT, Hawkins B, Lai FM (1989) IgA nephropathy associated with ankylosing spondylitis: occurrence in women as well as in men. Ann Rheum Dis 48: 435-437. Link: https://goo.gl/1rRKpu
- Andres HJ, Vielhauer V (2011) Renal co-morbidity in patients with rheumatic disease. Arthritis Res Ther 13: 222-229. Link: https://goo.gl/zPI3pu
- Nabokov AV, Shabunin MA, Smirnov AV (1996) Renal involvement in ankylosing spondylitis (Bechtrew’s disease). Nephrol Dial Transplant 11: 1172-1175. Link: https://goo.gl/bqrHEv
- Azevado DC, Ferreira GA, Carvalho MAP (2011) IgA nepdopathy in spondyloarthritis. Rev Bras Rheumatol 51: 104-108. Link: https://goo.gl/SLEUgW
- AzevedoI DC, Ferreira GA, Carvalho MAP (2011) IgA nephropathy in patients with spondyloarthritis followed-up at the Rheumatology Service of Hospital das Clínicas/UFMG. Rev Bras Rheumatol 51: 417-422. Link: https://goo.gl/221Jv2
- Jennete JC, Ferguson AL, Moore MA, Freeman DG (1982) IgA Nephropathy associated with seronegatve spondylarthropathies. Arthritis Rheum 25: 144-149. Link: https://goo.gl/WURxzV
- Esen B, Atay AE, Yildirim S, Sari H, Gökmen E, et al. (2014) IgA nephropathy in a patient with ankylosing spondylitis: A case report and mini report. Acibadem Universitesi Saglik Bilimieri Dergisi 4: 290-292. Link: https://goo.gl/VHBz16
- Van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis: A proposal for modification of the New York criteria. Arthritis Rheum 27: 361-368. Link: https://goo.gl/AMwGFl
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
