Rheumatica Acta: Open Access
Holistic Treatment of Inflammatory Disease for the Mobility Impaired
M Laymon* and K Laymon
Cite this as
Laymon M, Laymon K (2023) Holistic Treatment of Inflammatory Disease for the Mobility Impaired. Rheumatica Acta: Open Access 7(1): 001-005. DOI: 10.17352/raoa.000015Copyright
© 2023 Laymon M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Previous studies have demonstrated the positive impact of yoga and deep breathing exercises on overall physical wellness. The effects of deep breathing alone on metabolic rate, weight, and cholesterol management have not been studied well.
Objective: This study assessed changes in C-Reactive Protein (CRP) following a 60-day intervention of a 12-minute deep breathing program.
Methods: Sixty-six participants with a BMI >27 kg/m2 and 18 years - 70 years were enrolled in this study. Participants were assigned to either the control or intervention group in a single-blind manner. The intervention group followed the novel deep breathing program, while the control group did not modify their lifestyle or exercise. Anthropometric measurements and metabolic markers were evaluated and compared between the two groups after 60 days.
Results: After 60 days, the control group exhibited a mean increase change in CRP of 3.9%. The intervention group showed a mean reduction of CRP by 25%.
Conclusion: A guided daily 12-minute-deep breathing program can lead to reductions in inflammation marker CRP even without additional lifestyle modifications or medication modifications. Further investigations are warranted to explore the effects of novel deep breathing programs on metabolic markers and elucidate the underlying mechanisms of CRP reduction.
Introduction
C-Reactive Protein (CRP) is a protein biomarker that elevates with acute and chronic inflammation. Normal levels of CRP in healthy adults average 3 mg/L with levels of 8 mg/L to 10 mg/L considered high, and levels greater than 10 mg/L present an alarming pathological situation. Traditional inflammatory management approaches typically involve medications, modifications in dietary habits, physical activity, and behavior [1]. However, exploring additional adjunctive interventions, such as deep breathing exercises, to enhance the reduction of inflammatory markers has gained scientific interest. Deep breathing exercises are structured practices aimed at promoting relaxation, reducing stress, and improving overall well-being [2]. Deep breathing exercises are techniques that involve taking slow, deep breaths. Some studies suggest that practicing deep breathing exercises may promote weight loss, relaxation, and feelings of well-being. Additionally, some studies suggest that deep breathing exercises may help increase oxygen flow and improve metabolism, which could potentially aid in immune system efficiency. These programs emphasize the power of conscious breathing and teach individuals how to utilize their breath as a tool to manage their physical and mental states effectively. Deep breathing has been used for centuries in various cultures and is a fundamental component of practices like yoga, meditation, and mindfulness [3]. Mindful meditation and deep breathing exercises have been shown to improve the quality of life of individuals [4]. Deep breathing exercises encompass various techniques that emphasize diaphragmatic breathing, leading to increased oxygen intake and relaxation responses [5]. They have gained significant attention as a complementary approach to promote overall well-being and improve various aspects of health. The fundamental principle behind deep breathing programs is to engage in slow, deliberate, and controlled breaths, focusing on the inhalation and exhalation processes. By breathing deeply, individuals increase the intake of oxygen, which can have a range of positive effects on the body and mind [6]. Deep breathing stimulates the parasympathetic nervous system, responsible for the body’s rest-and-digest response, leading to a cascade of physiological changes that promote relaxation and stress reduction [7].
Common deep breathing techniques that are often incorporated into deep breathing programs include diaphragmatic breathing, box breathing, and 4-7-8 breathing among many other techniques [8-12]. Deep breathing programs can be practiced in various settings and formats, including individual sessions, group classes, online courses, or guided meditation apps [13]. These programs typically provide instructions on how to perform the techniques correctly and offer guidance on incorporating deep breathing into daily routines. They may also incorporate other elements such as visualization, body scan exercises, or progressive muscle relaxation to enhance the overall relaxation experience.
Deep breathing exercises, along with lifestyle modifications, have been shown to reduce inflammatory markers, increase basal metabolic rate, and promote weight loss. A novel breathing program has been suggested to produce similar results without modifications to lifestyle or diet. C-Reactive Protein (CRP) is used as a standard marker to measure bodily inflammatory response. Elevated CRP has been linked to chronic diseases such as cardiovascular disease, diabetes, cancer, and obesity. The conservative treatment for arthritis, diabetes, and obesity is weight-bearing activities and exercises such as walking, weightlifting, running, and swimming, etc. People who are mobility impaired are unable to participate in these activities. The objective of this study was to measure changes in CRP in subjects participating in a total of 12 minutes of daily deep breathing activities without incorporating exercises involving walking, running, swimming, or other aerobic activities.
Methods/study design
Study design
A single-blind randomized controlled trial where participants were assigned to either the control or intervention group.
Participants
A total of 66 volunteers, 30 in an intervention group and 36 in a control group, were recruited for the study. Participants who had a BMI greater than 27 and fell within the age range of 18 years - 70 years were selected for the study. Exclusion criteria included age below 18, BMI<27, participation in a weight loss program over the past three months, weight fluctuations over the past 3 months, participation in a weight loss program within the last three months, and subjects with pacemakers or taking Beta blocking medication.
Randomization
The participants were randomly assigned to either the control group or the intervention group. A block randomization process was employed to maintain balance and mitigate any differences between the groups in terms of gender, age, and BMI. This process involved dividing the participants into blocks based on these variables. Within each block, an equal number of participants were allocated to the intervention and control groups. The allocation sequence was generated using a computer-generated random number sequence.
Intervention
The control group did not change any lifestyle activities, especially diet, activity, or medications. The intervention group participated daily in 12 minutes of guided deep breathing exercises that were administered through an application that participants were required to log in to three times daily. Participants were not allowed to modify their diet or exercise behaviors during the trial period. Blood specimens for both groups were collected on days 0, 10, 30, and day 60 to evaluate CRP levels. Randomization: The participants were randomly assigned to either the intervention group or the control group. A block randomization process was employed to maintain balance and mitigate any differences between the groups in terms of gender, age, and BMI. This process involved dividing the participants into blocks based on these variables. Within each block, an equal number of participants were allocated to the intervention and control groups. The allocation sequence was generated using a computer-generated random number sequence. Blinding: A blinding protocol was implemented to ensure that the technicians performing measurements on the subjects remained unaware of which subjects belonged to the active group and which belonged to the control group. This blinding aimed to minimize potential biases during the measurement process. To maintain technician blinding, specific procedures were followed. Separate stations were established for subject check-in, which were physically separated from the measurement stations. This separation helped prevent the technicians from identifying the group allocation of the subjects while performing measurements. Intervention: The intervention group was instructed to participate in a 12-minute deep breathing program for the duration of 60 days. The deep breathing exercises were administered through a website, requiring participants to log in three times daily. The participants were provided with guided breathing exercises to follow during each session. The three sessions were divided into morning (10 minutes), afternoon (1 minute), and evening (1 minute). Specific times were dictated by everyone’s daily routines and meals (breakfast, lunch, and dinner) and therefore did not greatly interfere with daily routines.
At the start of the program, the active group (intervention) participants were provided login access to the website, which contained the deep breathing videos. Each participant had an individual account on the website used to intake initial survey questions and subject data integral to the intervention program. The website also logged the completed videos for each subject. The website was also used by the participants to log their water intake and provide input to additional survey questions prompted by the website while viewing the daily videos. They were instructed not to change other aspects of their lifestyle, including nutrition, eating habits, exercise, or other physical activities, to avoid confounding the benefits of the deep breathing program. The control group did not participate in the deep breathing program and maintained their usual lifestyle without any modifications to their diet or exercise behaviors during the trial period. Blood samples were collected on day 0 (baseline), day 10, and day 30 and analyses were conducted to evaluate metabolite concentrations including human growth hormone (HGH), total cholesterol, HDL, LDL, creatine, glucose, C-Reactive Protein (CRP), White Blood Cell (WBC) count, Hemoglobin (HGB), glycosylated hemoglobin A1C (HbA1C), blood CO2 and mean corpuscular hemoglobin (MCHC).
Statistical analysis
All statistical analysis was carried out using R version 4.1.1, with linear mixed modeling completed using the package lme4 (v1.1-31, [14]), and post-hoc testing using package emmeans (v1.8.5, [15]). Participants were categorized according to group (intervention vs. control) and time point (baseline, ten days, and thirty days following the intervention). Time was modeled as a continuous variable (number of days), with three-time points per participant. Covariates included participant age, sex, and baseline BMI. Models included a random effect of participants to correct for repeated measures and allow intercept variability. The Variance Inflation Factor (VIF) was calculated to ensure multicollinearity was negligible. For each model, residuals were tested using the Kolmogorov-Smirnov test for normality, as well as visually using QQ plots. In each outcome measure, outliers were removed at three standard deviations beyond the mean on a per-trial basis. One model was created per outcome measure, with FDR-adjusted p-values reported to correct for multiple comparisons. Post-hoc testing was completed using Tukey’s HSD to test the significance of changes over time for each group in outcomes with a significant group-by-time interaction. A p-value less than 0.05 was considered significant.
Results
CRP levels significantly decreased, indicating a positive response to the intervention.
The baseline CRP for the control and intervention groups were 9.11 mg/L and 10.68 mg/L respectively. At 60 days, the control group reported a net increase in CRP values of 0.36 mg/L, and the intervention group reported a net decrease in CRP values of 2.67 mg/L.
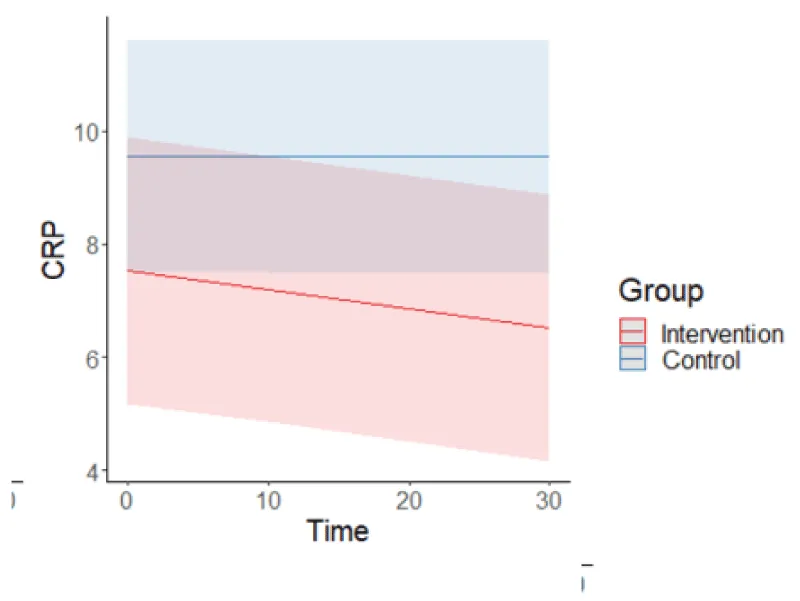
Table 1 represents each group by time interactions in outcome measures as significant, with post-hoc testing to demonstrate the significance of changes over time in each outcome for each group. Figure 1 graphically demonstrates the continued decline in CRP over the entire intervention period whereas, the control had no statistically significant change over the same period.
Although not the focus of this paper, other measures of metabolic activity were also significant such as the increase in Human Growth Hormone, decrease in HgA1c, decrease in cholesterol, fasting glucose, and creatinine.
Discussion
This study suggests that 12 minutes of daily deep breathing can promote a decrease in CRP Inflammatory markers in clinically overweight/sedentary patients without regard to lifestyle modifications. Recent studies have shown that deep breathing positively affects inflammation and oxidative stress in obesity [16-19]. Since deep breathing activities can be done by mobility-impaired/sedentary individuals and do not require weight-bearing, balancing, or strenuous activities, the incorporation of a deep breathing program for the mobility-impaired may decrease chronic inflammatory disease effects. Elevated CRP levels have been indicated in decreased mobility and may be used as a predictor of mobility decline [20-22]. Since many deep breathing programs are available free for download off the internet, this intervention may prove as an economical and non-medicinal intervention for the treatment of inflammatory disease that is readily available and appropriate for the mobility impaired. Further studies should evaluate deep breathing programs on the infirmed, paralytic, sedentary, and bedridden population. Since both groups did not change any lifestyle activities such as diet, schedules, and exercise; the decrease in CRP for the intervention group can only be attributed to the deep breathing activities. Elevated CRP is indicative of many different underlying pathologies [23-39]. It is therefore imperative that the underlying cause of the elevated CRP be determined so as not to miss early cancer, chronic bowel, acute infections, etc. This study did not investigate the underlying mechanisms through which deep breathing exercises affect CRP metabolism. Limitations of this study include not stratifying age groups (young, middle-aged, geriatric). Although efforts were made to blind the researchers, it was not possible to blind the participants themselves. This may have influenced their reporting of outcomes. Future studies could include assessments of more specific biomarkers, hormone levels, and physiological responses to provide a deeper understanding of the mechanisms involved. Further studies could also include pediatric, adolescent, and geriatric subgroups to elucidate groups that have greater response or need for incorporation of this type of intervention. Longitudinal studies that look at the sustainability of the observed effects and determine whether the benefits of the deep breathing program persist over time, continued response to an endpoint, duration of response, and the optimal amount of time per day of deep breathing to elicit the beneficial effects should also be conducted.
Conclusion
The results indicate that the deep breathing program had positive outcomes, demonstrating its potential to decrease markers of CRP in individuals. The intervention group, who participated in the deep breathing program, experienced a significant reduction in CRP over a 60-day period, while the control group showed no significant change in CRP. This study suggests that guided deep breathing exercises have the potential to contribute to reduced inflammatory processes in individuals, even in the absence of other lifestyle modifications. Future studies should address the limitations of this research by implementing a double-blind design, conducting longer-term investigations, examining a broader range of sedentary categories, and exploring the underlying mechanisms. By doing so, the effects of deep breathing exercises on inflammatory and immune responses can be better understood, paving the way for more targeted and effective interventions in rheumatic disease in the future.
This study was approved by the Touro University of Nevada Institutional Research Board. IRB Protocol #: TUNIRB000185.
All subjects signed an informed consent and were monitored for lifestyle and life event changes and compliance with the intervention for the intervention group.
The authors would want to thank Sean R. McWhinney, Department of Psychiatry, Dalhousie University, Halifax, NS, Canada for their assistance and expertise in statistical analysis of data.
- Cummings S, Parham ES, Strain GW; American Dietetic Association. Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2002 Aug;102(8):1145-55. doi: 10.1016/s0002-8223(02)90255-5. PMID: 12171464.
- Woodyard C. Exploring the therapeutic effects of yoga and its ability to increase quality of life. Int J Yoga. 2011 Jul;4(2):49-54. doi: 10.4103/0973-6131.85485. PMID: 22022122; PMCID: PMC3193654.
- Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Ann N Y Acad Sci. 2009 Aug;1172:54-62. doi: 10.1111/j.1749-6632.2009.04394.x. PMID: 19735239.
- Dada T, Mittal D, Mohanty K, Faiq MA, Bhat MA, Yadav RK, Sihota R, Sidhu T, Velpandian T, Kalaivani M, Pandey RM, Gao Y, Sabel BA, Dada R. Mindfulness Meditation Reduces Intraocular Pressure, Lowers Stress Biomarkers and Modulates Gene Expression in Glaucoma: A Randomized Controlled Trial. J Glaucoma. 2018 Dec;27(12):1061-1067. doi: 10.1097/IJG.0000000000001088. PMID: 30256277.
- Novotny S, Kravitz L. The science of breathing. IDEA Fitness Journal. 2007; 4(2): 36-43.
- Prpa M. Inhaling and exhaling: How technologies can perceptually extend our breath awareness. In Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems. 2020; 1-15.
- Jerath R, Crawford MW, Barnes VA, Harden K. Self-regulation of breathing as a primary treatment for anxiety. Appl Psychophysiol Biofeedback. 2015 Jun;40(2):107-15. doi: 10.1007/s10484-015-9279-8. PMID: 25869930.
- Hamasaki H. Effects of Diaphragmatic Breathing on Health: A Narrative Review. Medicines (Basel). 2020 Oct 15;7(10):65. doi: 10.3390/medicines7100065. PMID: 33076360; PMCID: PMC7602530.
- Ahmed A, Devi RG, Priya AJ. Effect of Box Breathing Technique on Lung Function Test. Journal of Pharmaceutical Research International. 2021; 33(58A): 25-31.
- Vierra J, Boonla O, Prasertsri P. Effects of sleep deprivation and 4-7-8 breathing control on heart rate variability, blood pressure, blood glucose, and endothelial function in healthy young adults. Physiol Rep. 2022 Jul;10(13):e15389. doi: 10.14814/phy2.15389. PMID: 35822447; PMCID: PMC9277512.
- Menhart S, Cummings JJ. The Effects of Voice Qualities in Mindfulness Meditation Apps on Enjoyment, Relaxation State, and Perceived Usefulness. 2022.
- Jayawardena R, Ranasinghe P, Ranawaka H, Gamage N, Dissanayake D, Misra A. Exploring the Therapeutic Benefits of Pranayama (Yogic Breathing): A Systematic Review. Int J Yoga. 2020 May-Aug;13(2):99-110. doi: 10.4103/ijoy.IJOY_37_19. Epub 2020 May 1. PMID: 32669763; PMCID: PMC7336946.
- Chaya MS, Kurpad AV, Nagendra HR, Nagarathna R. The effect of long term combined yoga practice on the basal metabolic rate of healthy adults. BMC Complement Altern Med. 2006 Aug 31;6:28. doi: 10.1186/1472-6882-6-28. PMID: 16945127; PMCID: PMC1564415.
- Bates D. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015; 67(1): 1-48.
- Lenth R. Estimated Marginal Means, aka Least-Squares Means. 2023.
- Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117-32. doi: 10.3390/ijms12053117. Epub 2011 May 13. PMID: 21686173; PMCID: PMC3116179.
- Battineni G, Sagaro GG, Chintalapudi N, Amenta F, Tomassoni D, Tayebati SK. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int J Mol Sci. 2021 Apr 30;22(9):4798. doi: 10.3390/ijms22094798. PMID: 33946540; PMCID: PMC8125716.
- Yong MS, Lee YS, Lee HY. Effects of breathing exercises on resting metabolic rate and maximal oxygen uptake. J Phys Ther Sci. 2018 Sep;30(9):1173-1175. doi: 10.1589/jpts.30.1173. Epub 2018 Sep 4. PMID: 30214120; PMCID: PMC6127488.
- Yau KK, Loke AY. Effects of diaphragmatic deep breathing exercises on prehypertensive or hypertensive adults: A literature review. Complement Ther Clin Pract. 2021 May;43:101315. doi: 10.1016/j.ctcp.2021.101315. Epub 2021 Jan 26. PMID: 33530033.
- Verghese J, Holtzer R, Lipton RB, Wang C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing. 2012 Jul;41(4):541-5. doi: 10.1093/ageing/afs038. Epub 2012 Mar 14. PMID: 22417984; PMCID: PMC3500856.
- Manrique-Espinoza B, Palazuelos-González R, Pando-Robles V, Rosas-Carrasco O, Salinas-Rodríguez A. Is there an association between inflammatory markers and lower physical performance in older adults? BMC Geriatr. 2022 May 7;22(1):403. doi: 10.1186/s12877-022-03091-7. PMID: 35525916; PMCID: PMC9077923.
- Sousa AC, Zunzunegui MV, Li A, Phillips SP, Guralnik JM, Guerra RO. Association between C-reactive protein and physical performance in older populations: results from the International Mobility in Aging Study (IMIAS). Age Ageing. 2016 Mar;45(2):274-80. doi: 10.1093/ageing/afv202. Epub 2016 Jan 28. PMID: 26822196.
- Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018 Apr 13;9:754. doi: 10.3389/fimmu.2018.00754. PMID: 29706967; PMCID: PMC5908901.
- Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001 Aug;38(2-3):189-97. doi: 10.1016/s0161-5890(01)00042-6. PMID: 11532280.
- Jimenez RV, Szalai AJ. Therapeutic Lowering of C-Reactive Protein. Front Immunol. 2021 Jan 29;11:619564. doi: 10.3389/fimmu.2020.619564. PMID: 33633738; PMCID: PMC7901964.
- Jutley GS, Sahota K, Sahbudin I, Filer A, Arayssi T, Young SP, Raza K. Relationship Between Inflammation and Metabolism in Patients With Newly Presenting Rheumatoid Arthritis. Front Immunol. 2021 Sep 28;12:676105. doi: 10.3389/fimmu.2021.676105. PMID: 34650548; PMCID: PMC8507469.
- Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, Kitas GD, Raza K. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 2013 Aug;65(8):2015-23. doi: 10.1002/art.38021. PMID: 23740368; PMCID: PMC3840700.
- Xu L, Chang C, Jiang P, Wei K, Zhang R, Jin Y, Zhao J, Xu L, Shi Y, Guo S, He D. Metabolomics in rheumatoid arthritis: Advances and review. Front Immunol. 2022 Aug 11;13:961708. doi: 10.3389/fimmu.2022.961708. PMID: 36032122; PMCID: PMC9404373.
- Zhu M, Ma Z, Zhang X, Hang D, Yin R, Feng J, Xu L, Shen H. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. 2022 Sep 19;20(1):301. doi: 10.1186/s12916-022-02506-x. PMID: 36117174; PMCID: PMC9484145.
- Markozannes G, Koutsioumpa C, Cividini S, Monori G, Tsilidis KK, Kretsavos N, Theodoratou E, Gill D, Ioannidis JP, Tzoulaki I. Global assessment of C-reactive protein and health-related outcomes: an umbrella review of evidence from observational studies and Mendelian randomization studies. Eur J Epidemiol. 2021 Jan;36(1):11-36. doi: 10.1007/s10654-020-00681-w. Epub 2020 Sep 25. PMID: 32978716; PMCID: PMC7847446.
- Li Y, Min L, Zhang X. Usefulness of procalcitonin (PCT), C-reactive protein (CRP), and white blood cell (WBC) levels in the differential diagnosis of acute bacterial, viral, and mycoplasmal respiratory tract infections in children. BMC Pulm Med. 2021 Nov 26;21(1):386. doi: 10.1186/s12890-021-01756-4. PMID: 34836530; PMCID: PMC8620633.
- Sakurai T, Saruta M. Positioning and Usefulness of Biomarkers in Inflammatory Bowel Disease. Digestion. 2023;104(1):30-41. doi: 10.1159/000527846. Epub 2022 Nov 18. PMID: 36404714; PMCID: PMC9843547.
- Wagatsuma K, Yokoyama Y, Nakase H. Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease. Life (Basel). 2021 Dec 10;11(12):1375. doi: 10.3390/life11121375. PMID: 34947906; PMCID: PMC8707558.
- Hua X, Dai JY, Lindström S, Harrison TA, Lin Y, Alberts SR, Alwers E, Berndt SI, Brenner H, Buchanan DD, Campbell PT, Casey G, Chang-Claude J, Gallinger S, Giles GG, Goldberg RM, Gunter MJ, Hoffmeister M, Jenkins MA, Joshi AD, Ma W, Milne RL, Murphy N, Pai RK, Sakoda LC, Schoen RE, Shi Q, Slattery ML, Song M, White E, Marchand LL, Chan AT, Peters U, Newcomb PA. Genetically Predicted Circulating C-Reactive Protein Concentration and Colorectal Cancer Survival: A Mendelian Randomization Consortium Study. Cancer Epidemiol Biomarkers Prev. 2021 Jul;30(7):1349-1358. doi: 10.1158/1055-9965.EPI-20-1848. Epub 2021 May 10. PMID: 33972368; PMCID: PMC8254760.
- Melnikov I, Kozlov S, Saburova O, Avtaeva Y, Guria K, Gabbasov Z. Monomeric C-Reactive Protein in Atherosclerotic Cardiovascular Disease: Advances and Perspectives. Int J Mol Sci. 2023 Jan 20;24(3):2079. doi: 10.3390/ijms24032079. PMID: 36768404; PMCID: PMC9917083.
- Badimon L, Peña E, Arderiu G, Padró T, Slevin M, Vilahur G, Chiva-Blanch G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front Immunol. 2018 Mar 2;9:430. doi: 10.3389/fimmu.2018.00430. PMID: 29552019; PMCID: PMC5840191.
- Nazemi P, SeyedAlinaghi S, Azarnoush A, Mabadi A, Khaneshan AS, Salehi M. Serum C-reactive protein greater than 75 mg/dL as an early available laboratory predictor of severe COVID-19: A systematic review. Immun Inflamm Dis. 2023 Dec;11(12):e1130. doi: 10.1002/iid3.1130. PMID: 38156391; PMCID: PMC10753867.
- Sproston NR, El Mohtadi M, Slevin M, Gilmore W, Ashworth JJ. The Effect of C-Reactive Protein Isoforms on Nitric Oxide Production by U937 Monocytes/Macrophages. Front Immunol. 2018 Jul 2;9:1500. doi: 10.3389/fimmu.2018.01500. PMID: 30013561; PMCID: PMC6036124.
- Schwedler SB, Kuhlencordt PJ, Ponnuswamy PP, Hatiboglu G, Quaschning T, Widder J, Wanner C, Potempa LA, Galle J. Native C-reactive protein induces endothelial dysfunction in ApoE-/- mice: implications for iNOS and reactive oxygen species. Atherosclerosis. 2007 Dec;195(2):e76-84. doi: 10.1016/j.atherosclerosis.2007.06.013. Epub 2007 Jul 31. PMID: 17669410.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
