Archives of Nursing Practice and Care
Effect of a Δ9-tetrahydrocannabinol (THC)/cannabidiol (CBD) formulation on cell monolayer viability and mitochondria integrity: Significance of the drug carrier/delivery system
Steven M Lipson*, Darcy Rodriguez, Heleen P Lipson and Ronald E Gordon
Cite this as
Lipson SM, Rodriguez D, Lipson HP, Gordon RE (2020) Effect of a Δ9-tetrahydrocannabinol (THC)/cannabidiol (CBD) formulation on cell monolayer viability and mitochondria integrity: Significance of the drug carrier/delivery system. Arch Nurs Pract Care 6(1): 042-048. DOI: 10.17352/2581-4265.000048Background: The increasing availability and abuse of recreational and medical cannabis has been associated with patients presenting with cyclical nausea, vomiting, intestinal cramps, and other manifestations, referred to as cannabinoid hyperemesis syndrome. An exact etiologic mechanism, especially on the cellular level, remains unclear. Continual studies are needed to address the effect of cannabinoid activity specifically addressing that which occurs in the gastrointestinal tract.
Methods: Cannabinoids in general, are immiscible in water-based cell culture media. Homogenization was performed to bring a THC/CBD formulation into a workable state for in vitro testing. The human colon adenocarcinoma (HT-29) epithelia cell line was used throughout this study. Mitochondrial cytotoxicity testing was performed using a fluorescein conjugated monoclonal antibody to the organelle’s outer membrane protein. Mitochondrial morphology was performed by transmission electron microscopy utilizing a commercially available cannabidiol product. Cell cultures, incubated with 50 to 125 µg/ml of a THC/CBD formulation for 72-h, were viewed daily for the appearance of a monolayer degeneration or a cytopathogenic effect.
Results: Cell monolayers treated with non-homogenized THC/CBD in phosphate-buffered saline or utilizing DMSO as analyte diluents, proved unremarkable. However, homogenization of the cannabinoid formulation at > 50 µg/ml affected a loss of monolayer confluency and mitochondrial membrane surface protein. Treatment of monolayers with CBD affected a loss of mitochondrial morphology as observed by an absence of organelle cristae and matrix structure. Mitochondrial loss of surface protein integrity occurred prior to monolayer degeneration.
Conclusion: A cytotoxic effect was realized in colon cell cultures following treatment with a cannabinoid liquid tincture formulation at concentrations > 50 µg/ml. The degenerative effect by the cannabinoid formulation was directed initially to a marked loss of mitochondrial morphology and loss of [organelle] surface integrity, followed by a monolayer degeneration. Critically, the immiscible THC/CBD analyte mandated a size distribution of lipid globules to single digit of smaller µM particulates.
Globule reduction was achieved by high speed homogenization. Additional studies in the animal model are needed to further address our findings in the search for an etiology to cannabinoid hyperemesis syndrome.
Abbreviations
THC: Δ9-Tetrahydrocannabinol; CBD: Cannabidiol; CHS: Cannabinoid Hyperemesis Syndrome; MT: Mitochondria; TEM: Transmission Electron Microscopy; IF: Immunofluorescence; PBS: Phosphate-Buffered Saline; DMSO: Dimethyl Sulfoxide; HT-29: Human Adenocarcinoma of the Colon
Introduction
The increasing availability and in turn, abuse of recreational (and medical) cannabis (marijuana) has been etiologically linked to patients presenting with but not limited to, cyclical diarrhea, vomiting, intense gastrointestinal cramps, and headaches – clinically referred to as cannabinoid hyperemesis syndrome or CHS [1]. Although once considered rare, CHS has markedly increased over the last number of years. Data obtained from two urban hospital Emergency Departments for example, showed 43% of 494 and 32.9% of 155 cannabis users (viz. 12 and > 20 day use/month, respectively) being diagnosed with CHS [2,3]. The inordinate use of cannabis is not considered life threatening, although two CHS-associated mortalities due to nonspecific electrolyte imbalances, have been reported [4].
The precise mechanism of CHS induction by THC consumption remains unclear [5]. However, some investigators have recognized a targeted effect by THC of mitochondrial physiologic disfunction using bronchopulmonary epithelium of the human lung (A549) cell cultures and pig brain neuronal mitochondria [6-8]. A need exists accordingly, to further investigate the effect(s) of cannabis or cannabis-containing products as etiologic agents of CHS, especially on the enteric cellular level.
The purpose of this study was to investigate the effect of a commercially available THC/CBD liquid tincture formulation on the viability of an intestinal epithelial cell line as well as mitochondrial integrity. As a model system, the human adenocarcinoma of the colon (HT-29) cell line was used throughout this study. Cellular toxicity was determined by a loss of HT-29 monolayer viability, mitochondrial (MT) membrane destruction, and organelle ultrastructural anomaly.
Materials and methods
Tetrahydrocannabinol (THC)/cannabidiol (CBD)
THC/CBD (medical marijuana sublingual liquid tincture) was purchased from Columbia Care, Inc., Brooklyn, NY (S. M. Lipson, NYS registry ID 1-753970197). The extracted Cannabis product, was fractionated in coconut oil medium-chain triglycerides (MCT), polysorbate-80, and ethanol, by the manufacturer as per trade secret. The extracted product consisted of a 20:1 sublingual tincture ratio of THC to CBD (Zero point 5ml of the stock preparation consisted of 5 mg THC and 0.25 mg CBD). Less than 5 ml of the commercial preparation was utilized in the performance of the technologic procedures described in this study.
Cannabidiol
Cannabidiol (CBD) was purchased at a concentration of 1.0 mg/ml in methanol, analytical standard, for drug analysis from Millipore-Sigma (St. Louis, MO.; Cat. No. C6395). Zero point 1, 1.6, and 6.3% of the CBD stock was prepared in cell culture maintenance medium wherein the lowest concentration approximated that of the CBD component of the medical marijuana sublingual liquid tincture formulation. The negative culture control consisted of maintenance medium but containing the same concentrations of methanol.
Dulbecco’s Phosphate-Buffered Saline (PBS)
Liquid filter sterilized PBS without calcium chloride and magnesium chloride was purchased from Millipore-Sigma (St. Louis, MO.; Cat. No. D8537).
Cell culture
The human carcinoma of the colon [Homo sapiens colon colorectal adenocarcinoma (HT-29) cell line was purchased from the American Type Culture Collection (ATCC-38TM; Bethesda, MD) and used throughout the study. Cells were grown in T25CM tissue culture flasks and passaged as per standard procedures. For mitochondria membrane antigen detection, testing was performed on monolayers which had been grown in 15 X 45 mm “1 Dram Vial Shell type 1 glass w/plug“ and containing 12 mm diameter cover slips. The growth medium (GM) consisted of Eagles’ minimal essential medium supplemented with 10% fetal bovine serum(FBS), 100 µg/ml streptomycin, 100 units penicillin, 1% L-glutamine, and 1% amphotericin B. Maintenance medium (MM) was identical to the GM except that 2% FBS was used in the medium supplement.
Immunofluorescence
The immunostain consisted of an anti-mitochondrial clone 113-1 Alexa Fluor® 488 conjugate mouse monoclonal antibody recognizing the 60 kDa non-glycosylated surface component of the intact mitochondria organelle (Cat. # Mab1273A4, Millipore-Sigma (St. Louis, MO).
Dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) was purchased from Millipore-Sigma (Cat. No.W387520). The THC/CDB formulation was suspended in 10 and 20% DMSO (in PBS) to affect concentrations of 50, 64, 100, and 125 µg/ml. Zero point 25 µl of the THC/CBD concentrations were inoculated in triplicate onto monolayers of HT-29 cells grown in “shell vials.” Monolayers were observed and tested daily for a period of 72-h for any changes in culture confluency and in immunofluorescence signal, respectively. The control consisted of cultures treated with DMSO alone.
Homogenization
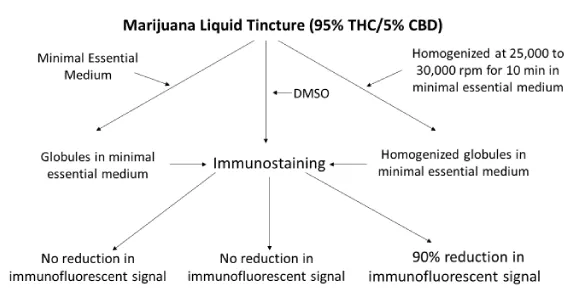
The THC/CBD liquid tincture formulation is immiscible in water based systems (viz. cell culture medium), precluding an accurate execution of in situ testing. Accordingly, THC/CBD lipid (fat) globules in MM were homogenized on ice to produce micro-size particles efficacious in the execution of the experimental protocol. The testing of THC/CBD at neat and increasing formulation concentrations both before and after homogenization was performed. Briefly, homogenization of THC/CBD suspensions were performed using an OMNI Homogenizer (Model TH; OMNI International, Kennesew, GA) at a speed of 25,000 to 30,000 rpm for 10 minutes. Particle size destruction was determined using a slide micrometer at 1 Div. = 0.01mM (AmScope, Los Angeles, CA). Briefly, 25ul of vortexed homogenized and non-homogenized THC/CBD formulations at 50ug/ml were placed onto the slide microgrid. After placement of a cover slip cover slip, the slip was read to determine the approximate diameter of both cannabinoid preparations. The slip was read at a total magnification of 400X.
Inoculation of cell cultures and determination of monolayer degeneration
The THC/CBD (cannabinoid) liquid tincture formulation was diluted in MM and DMSO from neat, 50, 64, 100, and 125 µg/ml for inoculation into previously seeded shell vials. Testing was performed using the cannabinoid formulation which has been suspended in homogenized and non-homogenized MM, and 10 and 20% concentrations of DMSO to determine the effect of the drug formulation on monolayer confluency and changes in fluorescence signal. Loss of MT morphology was addressed using the commercially purchased CBD. To avoid DMSO toxicity per se, 25 µl of each cannabinoid-DMSO suspension was inoculated onto monolayers routinely containing 200 µl MM. Cultures were incubated at 370C for 72-h and observed for daily for changes in monolayer confluency and fluorescence signal intensity. Controls consisted of MM and DMSO in the absence of the cannabinoid commercial formulation.
Detection of mitochondrial membrane protein
Shell vials were inoculated with concentrations of homogenized THC/CBD ranging from 50 to 125 µg/ml MM and incubated at 37oC for 72-h. Cover slips were washed with PBS, fixed with methanol, removed using a hypodermic needle, and affixed to glass slides using Permount histological glue (Lipson et al., 2017). The HT-29 monolayers were fixed in methanol, immunostained with antibody clone 113-1, and incubated for a period 37OC for 30 min. The monolayers were again washed and viewed under a Nikon eclipse E400 fluorescence microscope).
Transmission electron microscopy
Mitochondrial morphology was determined by transmission electron microscopy (TEM). Briefly, HT-29 cells were seeded into T-25cm tissue culture flasks at a concentration of 1 X 105 cells/ml according to standard procedures [9]. Upon the appearance of a 95% monolayer confluency, flasks were inoculated with increasing concentrations of CBD at 0.1, 1.6, and 6.3 µg/ml MM. After 24 and 48-h, monolayers were dispersed by trypsinization and fixed in 3% glutaraldehyde with 1% sodium cacodylate. The cells were dehydrated in ethanol, embedded en bloc, sectioned, and mounted on grids for examination using a Hatachi transmission electron microscope [10,11]. The negative control was treated as the experimental, but methanol was added to the MM instead of the CBD. Monolayers incubated with PBS only were tested as described above. The overall experimental design of this study is presented on Figure 1.
Results and discussion
The consumption of cannabis through ingestion or inhalation has been proposed to ameliorate pain/discomfort ranging from neoplastic diseases, administration of chemotherapy, neuropathy, inflammation, muscle spasticity and more [12,13]. Despite the absence of clinical trials, an increasingly large number of states have decriminalized the possession of marijuana, resulting in its recreational and/or medical use by the populace. Only three cannabis-containing drugs are currently FDA approved for clinical use. These drugs include EpidiolexR (containing CBD) for the treatment of two forms of childhood epilepsy (Dravet and Lennox-Gastaut Syndroms), dronabinol and nabilone (both containing THC) for the treatment of extreme loss of weight caused by AIDS, and nausea resulting from cancer chemotherapy, respectively [14]. Notwithstanding, long term daily or biweekly [recreational] use of cannabis, has been found to produce severe intestinal and related pathologic syndromes (e.g., CHS) opposite from that which the drug has been touted to remediate.
Two research groups have addressed in situ mitochondrial functionality by THC using A549 (human lung cancer) and the BeWo (human placenta choriocarcinoma) cell lines as model systems. Cannabis IC50 [toxic] concentrations of 7.5 µg/ml and 5 µM for A549 and BeWo cells respectively, had been reported [7,15]. However, due to those clinical manifestations recognized in CHS, it behooved us to investigate any potential cytotoxic activity imparted by THC to the in situ viability of HT-29 cells grown in monolayer culture. One of the more widely used cannabinoid products is the “sublingual liquid tincture” consisting of a 20:1 ratio of THC to CBD. Inasmuch as most studies have addressed the activity of pure system THC and to a larger extent CBD both in vivo and in situ/in vitro, it was our purpose to evaluate the combined THC/CBD commercial product but containing the higher ratio of THC to CBD [16,17].
Problematically, the commercially available THC/CBD formulation under investigation is immiscible in water-based systems, specifically the routinely used Eagle’s minimal essential medium utilized in our in vitro assay system. Importantly, biochemical/physiologic in vitro cell based assays commonly require analytes in a soluble form. Accordingly, experiments were performed to not only identify the effect of the cannabinoid formulation on cell viability and mitochondrial structural integrity, but to devise a system to address the immiscible state of the analyte in question.
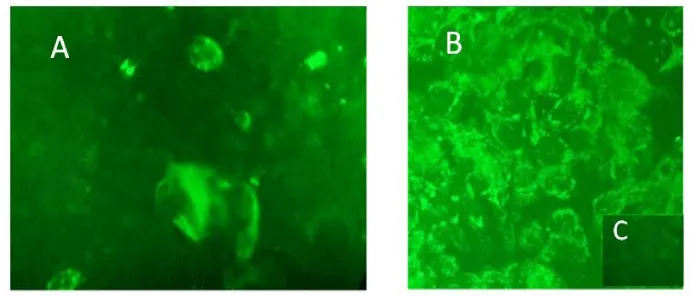
Dilution of THC/CBD liquid tincture in Eagle’s minimal essential medium (plus supplements) resulted in the formation of relatively large lipid globules. This effect was readily recognized following inoculation of the cannabinoid formulation into the HT-29 cells grown in monolayer culture (Figure 2). Dose response curves showed an increasing monolayer obfuscation at a cannabinoid formulation at >125 µg/ml, precluding a definitive reading of monolayers to determine cytotoxic activity (i.e., cellular granulation, loss of contact inhibition). Repeated washing of monolayers using PBS to remove beclouding concentrations of cannabinoid lipid globules proved ineffective. Notwithstanding, lower experimental cannabinoid inocula use in MM as the [drug] carrier/delivery system, proved relatively unremarkable.
A continued effort to bring the test analyte into suspension was approached using DMSO, a “universal” amphiphilic organic aprotic molecule capable of dissolving polar and non-polar molecules. The efficacy of DMSO had been recognized for decades in numerous disciplines including toxicology, pharmacology, molecular biology, cryopreservation, and as an enhancer of tissue penetration. Accordingly, DMSO was tested in our system as a carrier molecule for the THC/CBD liquid tincture formulation. Inoculation of HT-29 cells in monolayer culture with THC/CBD/DMSO preparations showed no monolayer degeneration nor loss of immunofluorescence signal. As alluded to above, our use of a markedly reduced final DMSO concentration in our assay system was not without consideration, as even single digit concentrations of the organic solvent had been shown to be toxic to metabolizing cells and might in turn, skew our findings [18-20].
The latest developments in therapeutic drug administration has spawned marked interest in the use of nanoparticle carriers such as, liposomes, polymeric micelles, and microspheres. Protocols using both chemical and physical methods have been shown effective in creating such nano-formulations, utilized in drug delivery systems [21,22]. It became apparent to us that a reduced particle (lipid globule) size distribution might be appropriate for use in our cell culture based assay system.
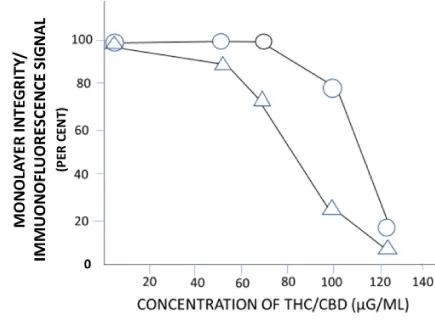
Further experiments were performed to address a potential loss of cell monolayer integrity and mitochondrial surface antigen following inoculation with a homogenized THC/CBD lipid globule suspension (Figures 2,3). Both unadulterated and homogenized cannabinoid preparations were tested at concentrations ranging from 50 to 125 µg/ml. Utilizing identical testing protocols as above, a continual loss of MT immunostaining and monolayer integrity commenced at a homogenized cannabinoid formulation > 50 µg/ml. Of note, the loss of MT 60 kDa membrane signal intensity and organelle structural integrity within our epithelial cell system proceeded a monolayer degeneration (Figure 4). TCH/CBD concentrations > 50 µg/ml in the non-homogenized MM control formulation failed to affect a clearly discernable monolayer degenerative effect or loss of immunofluorescence signal.
Particle size distribution of homogenized and non-homogenized THC/CBD preparations (in MM) were evaluated using a stage micrometer. Particle size distribution of non-homogenized preparations ranged from primarily 20 to 1500 µM in diameter. Homogenized preparations ranged from <10 to primarily single digit µM particles. We suggest that homogenized microscopic colloidal particles were able to attach and then traverse the eukaryotic cell membranes through a nonspecific endocytosis/pinocytosis [13]. Macromolecules of bacterial dimensions (0.2 to 2 µm in diameter and 2 to 8 um in length) for example, are capable of traversing the cellular membrane albeit in the prokaryote cell [23]. We do not suggest a THC/CBD attachment to HT-29 cellular membranes through a receptor mediated endocytosis.
The homogenization of THC/CBD lipid globules in suspensions not only affected a loss of monolayer integrity and MT surface antigen, but denoted the significance of an appropriate carrier/delivery system in our assay system. In support of our work, particle size reduction was effective in certain discrete [immunologic] cellular pathologies. Specifically, treatment of peripheral blood leukocyte cultures by sonicated polybrominated biphenyls or PBB (an environmental toxicant), negatively affected cell function while increasing immunoglobulin synthesis. Such findings presented a model system mimicking a hypergammaglobulinemia [24]. Preliminary testing of commercially prepared non-sonicated PBB proved benign. The immunologic work parenthetically, served as a laboratory component of that unique environmental catastrophe in Michigan, wherein the PBB fire retardant rather than the livestock nutritional supplement “Nutrimaster”, was inadvertently introduced into the food chain.
The decision to use maintenance medium (viz. Earle’s minimal essential medium plus supplements) rather than PBS in the preparation of our analyte was not without consideration. MM not only serves as a membrane stabilizing function and as an in situ [cell] nutritional source, but mediates particle-to-cell attachment. Specifically, HT-29 cells in monolayer culture and THC/CBD lipid globules in our assay system present with net electronegative surface charge potentials [25,26]. Monovalent and primarily divalent cation constituents in MM mediate a reduction of both monolayer and lipid globule electrostatic forces of repulsion [24,27]. The resultant latter effect is suggested to enhance microglobule-colon epithelial cell attachment through the actions of van der Waals forces and hydrogen bonding. Microparticle lipid globule passage through the eukaryotic cell membrane in turn, is proposed to occur through a clathrin-independent caveolin-mediated endocytosis [28,29]. The significance of a cation-mediate particle-to-cell attachment in our homogenized THC/CBD/MM transport or delivery system should not be underestimated.
One might ascribe those cation-mediated physicochemical surface effects through a reduction in diameter of the Stern-Guoy-Chapman electric double layer as proposed in the Derjaguin, Verwey, Landau, Overbeek (DVLO) theory [21,30,31]. Those interested in a detailed description and analysis of the DVLO/electric double layer paragon are referred to the concise overview by G. Trefalt and M. Borkovec, which is beyond the scope of study [32].
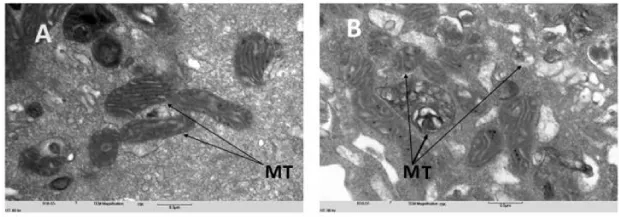
CBD in contrast to THC, is well tolerated, as doses of 700 and 1,500 mg/day among healthy volunteers showed no serious or minimal “side effects” [16,17]. It should be pointed out however, that such findings are not necessarily absolute. Complicating factors such as chronic diseases, patient age, body size, general constitution, and/or [in vivo] interactions between THC and CBD, may question one’s taking the liberty of biological extrapolations [33]. Notwithstanding, and in complement with our pure system study, it was our interest to additionally address the effect of CBD per se on MT integrity/morphology. As shown on Figure 5, CBD imparts a detrimental effect on MT morphology, as overtly evidenced by the absence of the organelles’ characteristic wrinkled cristae. MT membrane in folding parenthetically, serves the critical function of increased surface area, allowing optimal capacity for ATP generation (viz. electron transport, chemiosmosis) and in turn, cellular homeostasis.
A mechanism associated with our findings may be related to those events taking place in the endocannabinoid system (ECS). Specifically, the ECS is not only a constituent of the central and peripheral nervous systems, but is also present in organs and tissues throughout the body, including the gastrointestinal tract [34]. Importantly, cannabinoid CB1 (CB1R) and CB2 (CB2R) and potentially other receptors of the ECS bind to both endogenous [e.g., N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG)] as well as exogenous cannabinoids THC and CBD [35]. Inasmuch as the ECS contributes to homeostasis through a regulation of mitochondrial function, it would not be inappropriate to suggest a deregulation of the energy producing activity of the mitochondria and in turn, a detrimental effect on overall cellular integrity [36,37].
As a final point, it was not our intension to duplicate earlier studies addressing those adverse effects on MT bioenergetics imposed to either one or more cannabinoid constituents [6-8,38-42]. Our interest however, was directed to those potential morphologic aberrations of the MT and early stage dissolution of organelle membrane integrity within our enteric epithelial cell culture system. Perhaps of equal significance, is our recognition and execution of an appropriate transport/carrier system needed to investigate the effect of immiscible cannabinoids in an in vitro water-based cell culture assay.
Conclusions
Under the conditions of this study, a commercially available cannabinoid liquid tincture formulation and CBD per se, proved detrimental to the viability of HT-29 cells grown in monolayer culture and mitochondrial morphologic integrity, respectively. Significantly, and at an experimental dosing regimen from 50 to 125 µg/ml, the THC/CDB formulation affected a loss of MT antigen/immunofluorescent signal prior to the appearance of a cell monolayer degeneration. On the ultrastructural level, CBD alone affected an anomalous MT morphology, overtly recognized by an absence of organelle membrane in-folding and in turn, the characteristic cristae. It would not be unreasonable accordingly, to suggest a cannabinoid- and/or cannabidiol-associated deregulation of MT activity and in turn, the cytotoxic effect.
The THC/CBD liquid tincture formulation is immiscible in water-based systems. Homogenization of THC/CBD-associated lipid globules yielded a carrier/transport system detrimental to the viability of our cell culture system. We suggest a potential entry of homogenized microparticles into metabolizing HT-29 cells resulting in an organelle dysfunction followed by a cellular degeneration.
Our study, on the in-vitro level, draws attention to a potential link between cannabis intake and gastrointestinal dysfunction reflective of CHS. The in vitro use of the HT-29 cell line as a model system directs one’s attention to a potential mechanism associated with CHS/bowel dysfunction on the organismic level. Our work further points out the critical need to recognize and address any potential assay limitations (e.g., analyte immiscibility), which might adversely affect a given test’s negative predictive value. Further studies are needed in the animal model to further address the mechanism(s) of cannabinoid activity on gut physiology.
Declarations
SL and RG conceived and designed the research. SL, DR, and HL conducted the experiments. RG and SL performed the electron microscopy and analyzed the data. SL wrote the manuscript. All data was generated in-house, and no paper mill was used. All authors read and approved the manuscript. No human subjects were involved in the experimentation. The authors acknowledge that there are no conflicts of interest.
Funding
This work was supported in part, by a St. Francis College Faculty Research Grant and funding from the Department of Biology and Health Sciences. We appreciate the technical assistance of Kirsten Casares.
- Grewal JK, Loh LC (2020) Health considerations of the legalization of cannabis edibles. Can Med Assn J 192: E1-E2. Link: https://bit.ly/34tm7Gc
- Habboushe J, Rubin A, Liu H, Hoffman RS (2018) The Prevalence of Cannabinoid Hyperemesis Syndrome Among Regular Marijuana Smokers in an Urban Public Hospital. Basic Clin Pharmacol Toxicol 122: 660-662. Link: https://bit.ly/3nvAMJZ
- Hernandez JM, Paty J, Price IM (2018) Cannabinoid hyperemesis syndrome presentation to the emergency department: A two-year multicentre retrospective chart review in a major urban area. Can J Emer Med 20: 550-555. Link: https://bit.ly/3jENUdq
- Nourbakhsh M, Miller A, Gofton J, Jones G, Adeagbo B (2019) Cannabinoid Hyperemesis Syndrome: Reports of Fatal Cases. J Forensic Sci 64: 270-274. Link: https://bit.ly/34EuNts
- Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA (2017) Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment -systematic review. J Med Toxicol 13: 71-87.
- Fisar D, Singh N, Hiroudova J (2014) Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol Lett 231: 62-71. Link: https://bit.ly/3loucmr
- Sarafian TA, Kouyoumjian S, Tashkin D, Roth MD (2002) Synergitic cytotoxicity of Δ9-tetrahydrocannabinol and butylated hydroxyanisole. Toxic Let 133: 171-179. Link: https://bit.ly/30Ku20P
- Sarafian TA, Kouyoumjian S, Khoshaghideh F, Tashkin DP, Roth MD (2003) Δ9-tetrahydrocannabinol disruptsmitochondrial function and cell energetics. 2003. Am J Physiol Lung Cell Mol Physiol 284: L298-L306. Link: https://bit.ly/30KyA7x
- Lipson SM, Ozen FS, Louis S, Karthikeya L (2015) Comparison of α-glucosyl hesperidin and epigallocatechin gallate on the Loss of Rotavirus Infectivity in Host Cell Culture. Front Microbiol 6: 359. Link: https://bit.ly/34ywHfj
- Hübner WGP, McNerney P, Chen BM, Dale RE, Gordon FYS, et al. (2009) Quantitative 3D Video Microscopy of HIV Transfer Across T Cell Virological Synapses. Science 323: 1743-1747. Link: https://bit.ly/3iHolqO
- Lipson SM, Gordon RE, Ozen FS, Karthikeyan L, Stotzky G (2011) Effect of Cranberry and grape juices on tight junction function and structural integrity among rotavirus-infected monkey kidney epithelial cell culture monolayers. Food Environ Virol 3: 46-54. Link: https://bit.ly/3iGcmtx
- Doheny K (2018) Can Marijuana Be The Answer For Pain? WedMD health News. Link: https://wb.md/36JVaRw
- Mayor S, Parton RG, Donaldson JG (2014) Clathrin-Independent Pathways of Endocytosis. Cold Spring Harb Perspet Biol 6: a016758. Link: https://bit.ly/36KfJxo
- McCareberg BH (2007) Cannabinoids. Their role in pain and palliation. J Pain Pallative Care Pharm 21: 19-28. Link: https://bit.ly/36JkFCo
- Anonymous (2019) Marijuana as Medicine. DrugFacts. Advancing Addiction in Science. NIH/NIDA. Link: https://bit.ly/3nt5L9l
- Lojpur T, Easton Z, Raez-Villanueva S, Laviolette S, Holloway AC, et al. (2019) Δ9-Tetrahydrocannabinol leads to endoplasmic reticulum stress and mitochondrial dysfunction in human BeWo trophoblasts. Reprod Toxicol 87: 21-31. Link: https://bit.ly/3lmAgvQ
- Cunha MJ, Carlini EA, Pereira AE, Ramos OL (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21: 175-185. Link: https://bit.ly/2SysUsK
- Bergamaschi MM, Queiroz RHC, Zuard AW, Cripa AS (2011) Safety and side effects of cannabidiol, a Cannabis sativa constituent. Cur Drug Safety 6: 237-249. Link: https://bit.ly/36HRzDv
- Galvao J, Davis B, Tilley M, Normando E, Duchen MR, et al. (2014) Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J 28: 1317-1330. Link: https://bit.ly/30Iosw5
- Tao J, Shing JT, Chow SF, Zheng Y (2019) Application of flash nanoprecipitation to fabricate poorly water-soluble drug nanoparticles. Acta Phama Sinica B 9: 4-18. Link: https://bit.ly/2SAS75T
- Verheijenb M, Lienhard M, Schrooders Y, Clayton O, Nudischer R, et al. (2019) DMSO induces drastic changes in human cellular processes and epigenetic landscape In Vitro. Sci Rep 9: 4641. Link: https://go.nature.com/3libxsu
- Waegele MM, LGunathunge CM, Li Ji, Li X (2019) How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J Chem Phys 151: 160902. Link: https://bit.ly/2Fa1Ixm
- Zhang S, Gao H, Bao G (2015) Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 9: 8655-8671. Link: https://bit.ly/2GNnI1o
- Tortora GJ, Funke BR, Case CL (2016) Microbiology. An Introduction. 12th Ed. p. 73. 2016; Pearson Education, Inc., New York.
- Joklik WK (1985) Virology. Chap. 4, The Virus Multiplication Cycle. p. 61. 1985; Appleton-Century-Crofts,Norwalk, CT.
- Lipson SM (1987) Effect of polybrominated biphenyls on the growth and maturation of human peripheral blood lymphocytes. Clin Immunol Immunopathol 43: 65-72. Link: https://bit.ly/3d8Ldhz
- Michen B, Graule T (2010) Isoelectric points of viruses. J App Microbiol 109: 388-397. Link: https://bit.ly/33DrZ0s
- Peters I, Trout GM (1945) The Influence of pH on the attraction between the fat globules and leucocytes of milk. J Dairy Sci 28: 283-289. Link: https://bit.ly/3lpLdg2
- Puck T, Sagik B (1953) Virus and cell interaction with ion exchangers. J Exp Med 97: 807-820. Link: https://bit.ly/34yOrY2
- Cohen AW, Hnasko, R, Schubert W, Lisanti MP (2004) Role of caveolae and caveolins in health and disease. Physiol Rev 84: 1341-1379. Link: https://bit.ly/3iI8RCR
- Hsiao I, Gramatke AM, Joksimoic R, Sokolowsk M, Gradzielsk M, et al. (2014) Size and Cell Type Dependent Uptake of Silica Nanoparticles. J Nanomed Nanotechnol 5: 248. Link: https://bit.ly/2F9KYpZ
- Bell JE, Miller C (1984) Effects of phospholipid surface charge on ion conduction in the K+channel of sarcoplasmic reticulum. Biophy J 45: 279-287. Link: https://bit.ly/2SDKnjs
- Wang HX, Zuo ZQ, Du JZ, Wang YC, Sun R, et al. (2016) Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines. Nanotoday 11: 133-144. Link: https://bit.ly/3lgPhPQ
- Trefalt G, Borkovec M (2014) Overview of DLVO Theory. Laboratory of Colloid and Surface Chemistry (LCSC), University of Geneva. Licensed under the Creative Commons Attribution 4.0 International License. Link: https://bit.ly/2GuyY3g
- Lucas CJ, Galettis P, Scneider J (2018) The pharmacokinbetucs and the pharmacodynamics of cannaninoids. Brit J Clin Phar 84: 2477-2482. Link: https://bit.ly/3lmM0yr
- Pesce MA, D'Alessandro O, Borrelli S, Gigli L, Seguella R, et al. (2018) Endocannabinoid‐related compounds in gastrointestinal diseases. J Cell Mol Med 22: 706-715. Link: https://bit.ly/34zSNhl
- Zou S, Kumar U (2018) Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci 19: 833-410. Link: https://bit.ly/36I1AjX
- Herst PM, Rowe, MR, Carson GM, Berridge MV (2017) Functional Mitochondria in Health and Disease. Front Endocrinol (Lausanne) 8: 296. Link: https://bit.ly/3iGuMdN
- Sallaberry CA, Astern L (2018) The endocannabinoid system, our universal regulator. J Young Invest 34: 48-55. Link: https://bit.ly/2Sy8EHQ
- Shrivastava A, Kuzontkoski PM, Groopman JE, Pras A (2011) Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol Cancer Ther 10: 1161-1172. Link: https://bit.ly/3jHjmrt
- Lipson SM, Karalis G, Karthikeyan L, Ozen FS, Gordon RE, et al. (2017) Mechanism of Antiviral Synergistic Activity by epigallocatechin gallate and the proanthocyanidin-containing the nutraceutical CystiCran-40. Food Environ Virol 9: 434-443. Link: https://bit.ly/30FXG7l
- Di Marzo, V, Piscitelli F (2015) The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 12: 692-698. Link: https://bit.ly/30J5XHE

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
