Archives of Nursing Practice and Care
Improving measured health-related quality of life with outpatient high-dose methotrexate regimen among oncology patients with intracranial metastases: A systematic assessment
Heun Min1*, Elizabeth Weil2, Maggie Nelson1, John Charlson1, Yee Chung Cheng1, Lubna N Chaudhary1, John Burfeind1, Janet Retseck1, Deepika Sriram1 and Sailaja Kamaraju1
2Department of Pharmacy, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Cite this as
Min H, Weil E, Nelson M, Charlson J, Cheng YC, et al. (2023) Improving measured health-related quality of life with outpatient high-dose methotrexate regimen among oncology patients with intracranial metastases: A systematic assessment. Arch Nurs Pract Care 9(1): 008-014. DOI: 10.17352/2581-4265.000065Copyright License
©2023 Min H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Intracranial metastases including leptomeningeal disease are not uncommon in patients with metastatic solid tumor cancers, such as breast and sarcoma. Treatment options are limited with disease progression on standard-of-care therapies, and high-dose Methotrexate (MTX) is offered for patients with well-preserved performance status. However, standard high-dose MTX treatment entails a lengthy hospitalization and close monitoring which can limit Quality of Life (QoL) for patients who already have multiple provider visits. The impact of high-dose MTX on patients’ daily lives has not yet been qualitatively examined. As a quality improvement project, our team designed an outpatient high-dose protocol for patients who tolerated at least one cycle of inpatient high-dose MTX, and herein we describe the protocol and a quality survey with patients’ feedback. The purpose of this study is to explore and compare the influence of high-dose MTX treatments in two different settings – inpatient and ambulatory – on patients’ QoL. Second, we aim to identify recurrent themes defining patients’ perceived QoL and healthcare experiences. This study identified key QoL impacts that high-dose MTX treatments have on metastatic breast and sarcoma patients. Patients experienced decreased health-related burdens and improved social and psychosocial well-being associated with high-dose MTX treatment compared to standard inpatient treatment. This study provides an opportunity to identify recurrent thematic domains defining QoL in women with metastatic breast cancer.
Introduction
Diffuse or multifocal Leptomeningeal Metastases (LM) are a common late manifestation of breast cancer, with rates of occurrences ranging between 10% - 42% [1,2]. Central nervous system metastases confer a dismal prognosis with a median survival of less than 1 year [2,3]. Methotrexate (MTX) is a chemotherapeutic agent active against breast and other primary cancers and has demonstrated Central Nervous System (CNS) penetrance at effective, cytotoxic concentrations against leptomeningeal metastases when given in high doses intravenously [4].
High-dose intravenous MTX (HD IV MTX) has been in use for several decades for the treatment of primary CNS lymphoma and for prophylaxis for patients at high risk for CNS involvement such as leukemia, lymphoma, and in the management of LM for breast cancer, and osteosarcoma [3,5,6]. MTX is commonly administered in inpatients over 2–4 hours with leucovorin rescue, vigorous IV hydration, and urine alkalinization [2,4]. Daily urine and serum sample collections are required to ensure cytotoxic MTX concentrations are attained and to determine the duration of supportive care therapy [6]. This rigorous inpatient HD IV MTX protocol imparts limitations when considering the required lengthy patient hospitalization for 3-10 days, patient comfort, and extensive hospital staff and resource utilization [7]. Furthermore, given that these patients have metastatic disease, quality of life, spending time with their loved ones, and symptom management take precedence over hospital stay [8]. To avoid prolonged hospitalizations for HD IV MTX, as a Quality Improvement (QI) project, we developed and implemented a regimen that would allow patients to receive outpatient HD IV MTX if they completed at least one successful inpatient treatment. This article describes the details of the regimen and qualitative survey with patients’ feedback.
Methods
Development of an outpatient methotrexate protocol
An outpatient HD IV MTX protocol indicated for breast cancer patients with CNS involvement was developed in collaboration with our oncology pharmacists, oncology nurses, and medical oncologists treating breast and sarcoma patients at Froedtert Hospital and Medical College of Wisconsin’s Cancer Center. Eligibility for this ambulatory protocol was determined upon receiving and tolerating at least one prior inpatient HD IV MTX treatment, confirmation of an adequate patient support system throughout the entirety of the outpatient regimen, and corroboration of the patient for education, adherence, and completion of treatment and supportive care.
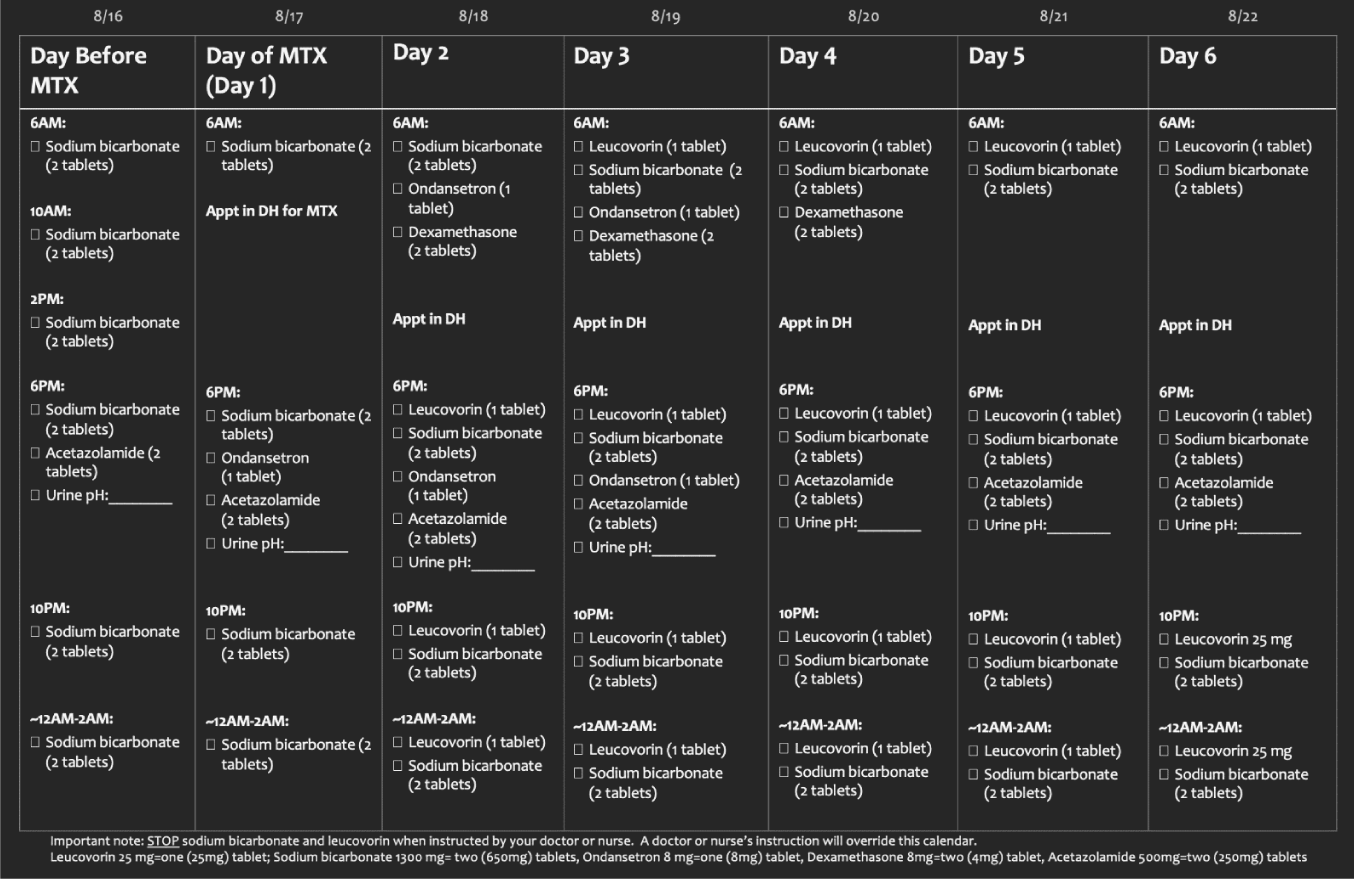
Prior to beginning the outpatient portion of this regimen, patients were required to have received an initial administration of HD IV MTX, 8 g/m2 in an inpatient setting, corresponding to Cycle 1. After completion of Cycle 1 patients who tolerated Cycle 1 and met the above criteria were eligible to transition to Cycle 2 via the outpatient HD IV MTX protocol if they preferred this approach. Patients underwent mandatory clinic teaching in person via clinic Registered Nurses (RN) and pharmacists (RPh) on home urine pH testing, required home supportive care medications, and patient responsibilities when/who to call with side effects or missed doses. Each patient was provided with individualized appointment calendars (Figure 1) that listed all appointment times, specific medication doses and times, and a place to record urine pH. Patients were provided with all medications, pH strips, and clinic RN/RPh contact information before initiation of Cycle 2. The Froedtert and the Medical College of Wisconsin 24-hour oncology clinic were notified of the patient’s name and planned HD IV MTX outpatient treatment start date, as they were the providers the patient was informed to contact during non-clinic hours.
What are the medications used for:
- Sodium Bicarbonate (fluids and pill): Increase urine pH to increase clearance of MTX from kidneys
- Acetazolamide: Increase urine pH to increase clearance of MTX from kidneys
- Leucovorin: Starts 24 hours after MTX to rescue healthy cells in the body
- Ondansetron: Used to prevent nausea
- pH strips: Used to test the urine pH.
If urine pH fell between 6-7, patients were instructed to take an extra dose of prescribed oral acetazolamide and sodium bicarbonate and repeat urine pH testing in one hour. If the pH returned a reading above 7, patients were to resume the normal course of therapy. However, if the pH reading remained below 7, patients were instructed to immediately call and come into the Froedtert and the Medical College of Wisconsin oncology 24-hour clinic; however, the patient returned daily to the infusion center for supportive care and labs. During these visits, the supportive care regimen was reinforced. Patients were coached and encouraged to utilize their clinic-provided home to-do sheets and check off all items prior to returning to the infusion clinic the next day. Clinic staff were instructed to contact and page the patient’s physician if the patient was unable to follow and complete all items on the supportive care calendar. New home checklists were reviewed in detail with each patient at the end of every day visit to adequately prepare for next-day treatments. Daily verifications of the next infusion appointment dates and times were conducted as well.
One day before beginning Cycle 2, patients were instructed to begin 1,300 mg (two 650 mg tablets) of sodium bicarbonate orally every four hours and one dose of acetazolamide 500 mg (two 250 mg tablets) and check urine pH as baseline at home. Although acetazolamide is not used as our standard of care in the inpatient setting for urine alkalization, it was added in the outpatient setting to ensure urine alkalinization at home, since patients would have fewer urine pH checks and less availability to sodium bicarbonate bolus at home for low urine pH. The responsibilities of ambulatory oncology pharmacist were contacting the infusion center to pre-process sodium bicarbonate, and clinic nurses contacting patients to ensure initiation of oral sodium bicarbonate and confirming patients were adequately supplied with required supportive care medications and supplies: sodium bicarbonate, acetazolamide, ondansetron, dexamethasone, leucovorin, and pH strips.
Day 1 (D1) of Cycle 2 began upon the arrival of the patient at the DH at 08:00 AM. Sodium bicarbonate infusion for a duration of 3 hours was commenced immediately at 08:00 AM since it was pre-processed the day before. With initial infusion, standard labs were drawn, Liver Function Tests (LFTs), and complete blood count with differential (CBC w/ diff) and Serum Creatinine (SCr), respectively. Urine pH was obtained 2 hours into the sodium bicarbonate infusion. If the urine pH was above 7, two hours into the infusion, at 10:00 AM, the 8 g/m2 HD-MTX infusion was initiated. At this time, patients’ 2L sodium bicarbonate 24-hour continuous home pumps were installed and begun. One hour into the MTX infusion, urine pH was repeated. Upon completion of two hours of continuous HD IV MTX infusion, at 12:00 PM, patients were provided with individualized home-to-do sheets demarcating proper home medications, urine pH monitoring instructions, necessary contact information, and confirmation of the next appointment time. Specific oral medications provided on D1 consisted of ondansetron indicated for nausea and sodium bicarbonate (two 650 mg tablets (1300 mg) every 4 hours while awake) and acetazolamide (two 250 mg tablets (500 mg) once nightly for urine alkalization. All patients were left with a continuous infusion of sodium bicarbonate given via a home infusion pump, with the 2L bag carried in a provided backpack.
Day 2 (D2) Cycle 2 appointments were scheduled approximately one hour prior to when the MTX infusion was completed the day prior (approximately at 09:00 AM). Upon arrival, a sodium bicarbonate bolus was administered. After completion of the bolus, the 24-hour home sodium bicarbonate bag was exchanged with a new bag. Patient labs were drawn timed exactly 24 hours after the completion of the MTX infusion on D1 and included LFTs, SCr, CBC w/ diff, urine pH, and an MTX level. Exactly 24 hours after the completion of the MTX infusion IV leucovorin rescue was also initiated beginning with once IV bolus dose of at the infusion center for 50 mg of leucovorin. Like day 1 patient instruction, patients were provided with individualized home to-do sheets, and appointment times were reviewed. New medications provided on D2 consisted of leucovorin 25 mg to be taken orally every four hours as an MTX rescue and dexamethasone 8 mg every morning for nausea. Previously mentioned supportive care of ondansetron, sodium bicarbonate, and acetazolamide was continued. Patients were asked to continue urine pH monitoring once per night and follow the instructions outlined above for any pH below 7.
Day 3 (D3) appointments were scheduled approximately one hour prior to when MTX was completed on D1 (approximately at 09:00 AM). Upon arrival at the DH, patients were started on sodium bicarbonate bolus and IV leucovorin (no specific timing for IV leucovorin was required for D3 and beyond). Standard labs outlined above and a timed MTX level were drawn exactly 48 hours after HD IV MTX infusion had been completed. Home sodium bicarbonate bags were exchanged upon bolus completion and patients were instructed to continue leucovorin, ondansetron, dexamethasone, sodium bicarbonate, acetazolamide, and urine pH monitoring as outlined above.
Contrary to D1-D3, appointments scheduled for day 4 (D4) and beyond were scheduled based on patient-preferred availability as MTX labs and IV leucovorin did not have to be drawn and administered time-specifically, respectively. Upon patient arrival, sodium bicarbonate bolus was begun, non-timed MTX levels and standard labs were drawn, and IV leucovorin was administered. Home infusion sodium bicarbonate bags were exchanged and patient instructions on continuing leucovorin, ondansetron, dexamethasone, sodium bicarbonate, acetazolamide, and urine pH monitoring were provided and reviewed.
During treatment parameters D2 and beyond, clinic RNs and staff were instructed to page patients’ physicians if any of the following lab abnormalities were apparent: LFTs > 10x upper limit of normal (ULN), SCr > 2x baseline and MTX levels > 10 µmol at 24 hours and > 5 µmol at 48 hours for further assessment, increased supportive care support, and possible admission to the hospital. Supportive care days were continued per the process outlined above for as long as the MTX level remained above 0.1 µmol.
During treatment parameters D2 and beyond were outlined for clinic RNs and staff to page patients’ physicians when the MTX level was less than 0.1 µmol. At that time, if the patient was feeling well, supportive care was discontinued because the patient had met the criteria for clearing MTX.
Study design
This Quality Improvement (QI) project was conducted in a single center, Froedtert Hospital and Medical College of Wisconsin, Department of Hematology, between January 2021 and March 2022. Given that the project provided clinical care for and impacted patient care directly without selection into intervention and control groups, this study met the criteria for a QI project and therefore received an exemption from formal IRB review.
Study population and recruitment
A sampling of patients meeting eligibility criteria (age equal to or greater than 18 years old, solid tumor diagnoses with intracranial (IC) ± Leptomeningeal Metastasis (LM), disease progression on standard of care treatment, successful transition from inpatient to outpatient HD IV MTX treatment protocol within the past 12 months) was completed at Froedtert Hospital and Medical College of Wisconsin.
For eligible patients upon consent, qualitative semi-structured phone interviews were conducted with a focus on physical functioning and symptom burden. Thematic analysis was utilized.
Study procedures and variables
Informed consent was obtained from eligible study participants prior to performing any study procedure and questionnaire.
To assess patient HRQOL, qualitative semi-structured phone interviews were conducted with a focus on physical functioning and symptom burden. Interviews consisted of an institutionally developed questionnaire that discloses patients’ experiences with both inpatient and outpatient HD IV MTX treatments and assesses underlying patient preferences and reasoning for an inpatient or outpatient HD IV MTX protocol. A series of questions were also used to evaluate symptom burden, toxicities, conveniences and barriers, patient satisfaction, and patient-encountered challenges during the course of their inpatient and outpatient HD IV MTX therapies. The full interview questionnaire developed can be found in the supplementary section.
The functional status of participants was quantified via the Eastern Cooperative Oncology Group (ECOG) Scale of Performance Status. Demographic data and medical history were also collected through participant interviews.
Thematic assessment
The sociodemographic characteristics of the patient sample were described using a distribution of percentages. The content of individual questionnaire answers was analyzed for consistent patterns or recurrent motifs to derive themes commonly reinstated in participant responses. Participants’ perception of their respective Qualities of Life (QoL) identified by the open-ended questionnaire was also examined.
Results
Study population characteristics
Of the 10 patients who were screened, three patients were eligible, and thus included in the study: 3 (100%) women; mean age was 52 years; primary cancer diagnoses were breast cancer (67%) and sarcoma (33%); 3 (100%) patients had received prior whole brain radiation therapy (WBRT) and targeted therapy. Previous lines of treatment received by study patients were 3 ± 1. Patients presented with tumor stage IV (100%) and all patients were symptomatic but completely ambulatory (100%); all 3 (100%) patients had previous medical conditions: hypertension, dyslipidemia, and/or hypothyroidism (67%), and depression and anxiety (33%). Demographic and clinical characteristics of the study population are summarized in Table 1.
Data analyses: QoL indices and predictors
Eligible subjects were contacted via phone communication to schedule and complete the interview questionnaire. All patients included in the study (N = 3; breast cancer = 2, sarcoma = 1) answered the institutionally developed questionnaire. Interviews ranged from 30-60 minutes (mean 34.5min). All interviews were audio-recorded, transcribed, and checked for transcription errors. During and after interviews, notes were made to record the interviewee's reflections and the interviewer's thoughts.
Braune and Clarke’s six steps of thematic analysis were applied once all interviews were completed as follows: data familiarization, coding, searching for themes, reviewing themes, defining and naming themes, and writing a report. All interviews were audio-recorded, transcribed, and checked for transcription errors. During and after interviews, notes were made to record the interviewee’s reflections and the interviewer’s thoughts. Each interview was then coded and proofread for inconsistencies by two independent research members. Codes were then classified and organized into prospective themes with relevant interview data gathered. Inconsistencies of proposed preliminary themes were discussed, and more themes were further explored in lieu of the original data. The main themes were refined, defined, and named. The core, recurrent themes were presented via report, supported with verbatim quotations from patient interview responses.
The body of the interview consisted of questions designed to discern participants’ experiences while receiving inpatient and outpatient HD IV MTX treatments, respectively.
Upon completion of the six-step thematic analyses, recurrent themes were identified and discussed.
Study participants reported no differences nor worsening in functional burden or status upon completion of inpatient and outpatient HD IV MTX therapies. No subjects expressed any concern about receiving prompt communication or having any negative interactions with their physicians and hospital staff members.
Two patients (breast cancer = 1, sarcoma = 1) expressed the convenience of the availability of around-the-clock hospital staff and services provided during their inpatient HD IV MTX hospital stay.
It was convenient for my us [my husband and I] to have the option of calling them [hospital RNs] when needed…being able to call my nurse to help me use the restroom or go on a walk (when my husband was at work) was nice. (Participant 1)
My nurses were really helpful in helping me get up from bed, use the restroom when my family wasn’t around…I appreciated them [hospital RNs] for helping me collect my urine for “testing.” (Participant 3)
The majority of participants did not identify any functional differences while undergoing either HD IV MTX treatment regimens, but one subject (sarcoma = 1) reported fewer symptomatic episodes of nausea and emesis during outpatient HD IV MTX treatments when compared to more than 5 episodes experienced during inpatient HD IV MTX infusions.
All subjects were adherent to clinical instructions and appointment schedules provided by clinic staff and completed both Cycles 1 and 2. None reported difficulty in understanding and adhering to the clinic-provided patient education on appointment times, correct supportive care medication dosages, home urine pH monitoring, and recognition of red flag symptoms and urine pH values that must be reported to the Froedtert and Medical College 24-hour clinic urgently. No subjects mentioned any improvements that could have augmented their patient education experience. All subjects described having a secure system of transportation while navigating to and accessing their outpatient appointments and reported no additional barriers in travel.
Participants unanimously reported more comfortability and perceived convenience experienced while undergoing outpatient HD IV MTX treatments compared to their inpatient HD IV MTX experiences.
[I] felt more at ease being able to go home after each appointment at around noon and spend the rest of the day with my friends and family… and being able to carry on with errands for the day. (Participant 1)
One of the pitfalls of my first cycle [treatment cycle] was that I was confined to my (hospital) bed all day for days. Sure, I was able to walk around, get up from my bed but having an IV in you 24/7 and having to stay overnight for days in the hospital was unpleasant, to say the least…Overnight hospital stays also placed a burden on my husband who had to commute back to the hospital after work instead of going home directly…[Patient reflection on outpatient treatments] It was real convenient being able to go home after my IVs after a couple of hours. I personally didn’t mind checking my urine samples at home nor did I find it particularly difficult. (Participant 2)
The staff were lovely, and I had no qualms during the entirety of my inpatient stays. However, I really appreciated being able to go home just after a few hours after receiving the medication. I’d spent countless nights in the hospital for my previous cycles. Going home, sleeping in my own bed, spending time with my cats and husband and friends, being able to make it to my other appointments are some of the many positives I can think of on the top of my head. (Participant 3)
Thematic analyses of interview responses showed recurrent premises of more convenience, improved personal and family time, and stronger emotional support when asked about their outpatient protocol experience in comparison with their inpatient. All participants overtly disclosed their preference for the outpatient HD IV MTX protocol, distinguishing patient autonomy and nonobligatory hospitalization associated with the outpatient regimen as two QoL values that influenced their partiality.
Discussion
Our QI project suggests that the use of HD IV MTX protocol is feasible in outpatient settings for solid tumor patients. Indeed, after completing treatments in an outpatient setting, study participants reported enhanced satisfaction and positive changes in attitude and outlook. Patients reported increased awareness of symptoms and daily energy patterns and proactive engagement in maintaining daily medications and urine pH monitoring. Understandably, our patients reported the aforementioned self-awareness and increased availability of personal time allowing them to plan and structure their daily routines that maximized their Health-Related Quality of Life (HRQOL) and general happiness. The execution of an outpatient HD IV MTX infusion required the development of a highly detailed protocol as outlined above to safely provide a very toxic medication in the outpatient setting [2,4]. Education for the staff and the availability of a 24-hour oncology clinic at Froedtert and the Medical College campus which was aware of the patient’s case in the event it was needed was essential. Selection of appropriate patients who met our inclusion criteria tolerated C1, and were agreeable to extensive education regarding supportive care and what to do in the event that they were experiencing side effects, had issues with their urine pH, or their home sodium bicarbonate pump was necessary to safely provide this care. Due to the extreme detail and education that was provided to the staff and patients, no patients required this additional resource.
HRQOL assessment in cancer patients has become an important factor in determining treatments that not only measure success in terms of overall survival or progression-free survival but also in terms of HRQOL improvement [9,10]. Although Patient-Reported Outcome (PRO) instruments are cancer or symptom-specific, there is currently no extensive data regarding HRQOL in primary solid tumor patients with IC metastases [11]. Although the cohort size is small, our feasibility QI study revealed a beneficial role of QoL assessments in improving clinical practice through more inclusive decision-making with patient input and identifying key measures valued by patients during a course of treatment [6,7,12-14]. Analyses of patient responses revealed these features provided patients with more perceived convenience and happiness and less emotional burden. While completing outpatient treatments, participants described feeling less helpless and more confident in coping with symptoms. Patients reported having increased motivation to take ownership of their appointments and general health monitoring. Subjects reported the outpatient HD IV MTX protocol provided them with more independence and a sense of control over their disease as they were relegated to specific responsibilities within their outpatient treatment protocols. Patients’ adherence to clinic instructions and daily ambulatory DH appointments was very high, indicating its feasibility.
Limitations
This study should be interpreted in the context of two limitations. First, this study was conducted at a single institution cancer center with a small subgroup of patients limiting generalizability. Second, we selected to create our own open-ended questionnaire to assess the entirety of patient experiences in patients’ own words and not limited by preformed phrases, numerical scales, or granular questionnaires that detail particular symptoms. Moreover, we elected to use an institutionally developed questionnaire that was exclusive to an HD IV MTX protocol.
One limitation of this study arises from the observational cross-sectional design of patients from only one institution, Froedtert Hospital and Medical College of Wisconsin. As we interviewed a small number of eligible study participants, this may limit the generalizability of this study and hence, cannot represent the full diversity of patient experiences. A formal cost-utility analysis was not performed in this study given modest resources and a limited study pool. Data on cancer-related occupational and financial challenges that caregivers may have faced throughout patient treatments were not collected in this study. Hence, future directions for further study will be to identify potential correlations between treatment preferences and financial and caregiver inputs.
Conclusion
In the context of an evolving healthcare delivery system where patient-centeredness is prioritized, actively incorporating patient-reported QoL-enhancing measures in the development of treatments may improve experience, efficiency, and outcomes of care. Given our favorable patient responses to an outpatient HD IV MTX protocol, continued utilization of an outpatient protocol versus the traditional inpatient approach and further work to refine the outpatient experience should be prioritized.
Slowing disease progression and increasing median survival are critical goals of oncology therapy. However, treatment aspects of comfortability, patient emotional and mental health, and stress burden have not yet been explored, especially in patients with LMD. Traditional therapies of inpatient HD IV MTX can be burdensome due to lengthy hospital stays, a decrease in patient independence, limited time with loved ones, and an added encumbrance of caregivers. Our study identified core, patient-reported QoL measures that an outpatient HD IV MTX regimen offered, those being: patient autonomy, nonobligatory hospitalization, and more personal time. These values notably enriched patient comfort and emotional and mental well-being. An outpatient HD IV MTX regimen additionally empowered patients’ independence and awareness with personalized daily appointments and home to-do lists.
- Santa-Maria CA, Cimino-Mathews A, Moseley KF, Wolff AC, Blakeley JO, Connolly RM. Complete radiologic response and long-term survival with use of systemic high-dose methotrexate for breast cancer-associated leptomeningeal disease. Clin Breast Cancer. 2012 Dec;12(6):445-9. doi: 10.1016/j.clbc.2012.07.010. Epub 2012 Sep 24. PMID: 23010203; PMCID: PMC4005330.
- Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, Hochberg F, Calabresi P, Egorin MJ. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998 Apr;16(4):1561-7. doi: 10.1200/JCO.1998.16.4.1561. PMID: 9552066.
- Kapke JT, Schneidewend RJ, Jawa ZA, Huang CC, Connelly JM, Chitambar CR. High-dose intravenous methotrexate in the management of breast cancer with leptomeningeal disease: Case series and review of the literature. Hematol Oncol Stem Cell Ther. 2019 Dec;12(4):189-193. doi: 10.1016/j.hemonc.2019.08.008. Epub 2019 Oct 14. PMID: 31629723.
- Tetef ML, Margolin KA, Doroshow JH, Akman S, Leong LA, Morgan RJ Jr, Raschko JW, Slatkin N, Somlo G, Longmate JA, Carroll MI, Newman EM. Pharmacokinetics and toxicity of high-dose intravenous methotrexate in the treatment of leptomeningeal carcinomatosis. Cancer Chemother Pharmacol. 2000;46(1):19-26. doi: 10.1007/s002800000118. PMID: 10912573.
- Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996 Aug;42(8 Pt 2):1322-9. PMID: 8697606.
- Wayne K. Preventing Methotrexate Toxicity: Know How to Use Leucovorin, Glucarpidase. Oncology Pharmacist. 2013.
- Bazan F, Dobi E, Royer B, Curtit E, Mansi L, Menneveau N, Paillard MJ, Meynard G, Villanueva C, Pivot X, Chaigneau L. Systemic high-dose intravenous methotrexate in patients with central nervous system metastatic breast cancer. BMC Cancer. 2019 Nov 1;19(1):1029. doi: 10.1186/s12885-019-6228-6. PMID: 31675937; PMCID: PMC6823971.
- Scott BJ, Oberheim-Bush NA, Kesari S. Leptomeningeal metastasis in breast cancer - a systematic review. Oncotarget. 2016 Jan 26;7(4):3740-7. doi: 10.18632/oncotarget.5911. PMID: 26543235; PMCID: PMC4826166.
- Oh HS, Menéndez ÁF, Santos VS, Martínez ÁR, Ribeiro FF, Vilanova-Trillo L, Figueiras MC, Ferreiros MP. Evaluating health related quality of life in outpatients receiving anti-cancer treatment: results from an observational, cross-sectional study. Health Qual Life Outcomes. 2021 Oct 18;19(1):245. doi: 10.1186/s12955-021-01876-9. PMID: 34663356; PMCID: PMC8524828.
- Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin. 2016 Jul;66(4):309-25. doi: 10.3322/caac.21341. Epub 2016 Feb 26. PMID: 26919165.
- Turner-Bowker DM, Hao Y, Foley C, Galipeau N, Mazar I, Krohe M, Globe D, Shields AL. The use of patient-reported outcomes in advanced breast cancer clinical trials: a review of the published literature. Curr Med Res Opin. 2016 Oct;32(10):1709-17. doi: 10.1080/03007995.2016.1205005. Epub 2016 Aug 9. PMID: 27331272.
- Bottomley A, Reijneveld JC, Koller M, Flechtner H, Tomaszewski KA, Greimel E; 5th EORTC Quality of Life in Cancer Clinical Trials Conference Faculty. Current state of quality of life and patient-reported outcomes research. Eur J Cancer. 2019 Nov;121:55-63. doi: 10.1016/j.ejca.2019.08.016. Epub 2019 Sep 24. PMID: 31561134.
- Pe M, Dorme L, Coens C, Basch E, Calvert M, Campbell A, Cleeland C, Cocks K, Collette L, Dirven L, Dueck AC, Devlin N, Flechtner HH, Gotay C, Griebsch I, Groenvold M, King M, Koller M, Malone DC, Martinelli F, Mitchell SA, Musoro JZ, Oliver K, Piault-Louis E, Piccart M, Pimentel FL, Quinten C, Reijneveld JC, Sloan J, Velikova G, Bottomley A; Setting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data Consortium (SISAQOL). Statistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic review. Lancet Oncol. 2018 Sep;19(9):e459-e469. doi: 10.1016/S1470-2045(18)30418-2. PMID: 30191850.
- Clarijs ME, Thurell J, Kühn F, Uyl-de Groot CA, Hedayati E, Karsten MM, Jager A, Koppert LB. Measuring Quality of Life Using Patient-Reported Outcomes in Real-World Metastatic Breast Cancer Patients: The Need for a Standardized Approach. Cancers (Basel). 2021 May 12;13(10):2308. doi: 10.3390/cancers13102308. PMID: 34065805; PMCID: PMC8151772.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
