Archives of Sports Medicine and Physiotherapy
The effect of exercise preconditioning with high-intensity interval training on cardiac protection following induction of myocardial infarction through mitochondrial dynamic changes in cardiac tissue in male rats
Azam Ahmadi1*, Majid Kashef1, Hamid Rajabi2 and Mojtaba Salehpour1
2Department of Exercise Physiology, Faculty of Physical Education & Sport Sciences, Kharazmi University, Tehran, Iran
Cite this as
Ahmadi A, Kashef M, Rajabi H, Salehpour M (2023) The effect of exercise preconditioning with high-intensity interval training on cardiac protection following induction of myocardial infarction through mitochondrial dynamic changes in cardiac tissue in male rats. Arch Sports Med Physiother 8(1): 011-018. DOI: 10.17352/asmp.000018Copyright
© 2023 Ahmadi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Exercise training prevents the adverse effects of Myocardial Infarction (MI) and Ischemia/Reperfusion (I / R) and it seems that mitochondria have an important role in exercise-induced cardioprotection. So, the purpose of this study was to investigate the effects of exercise preconditioning with 4 weeks of High-Intensity Exercise Training (HIIT) on cardiac damage and mitochondrial dynamic proteins as effective factors in cardiac protection following MI. Twenty Male Wistar rats were randomly divided into 4 groups HIIT + MI, MI, HIIT, and Control. Training groups performed 4 weeks (5 days per week) of high-intensity interval training. HIIT protocol consisted of 10*1min running intervals that were separated by 2 min rest. Training intensity varied every week. For induction of myocardial infarction, a subcutaneous injection of isoproterenol was used. Creatine Kinase (CK) and lactate Dehydrogenase (LDH) were measured in serum and Drp1, and Mfn2 gene expression were measured by the real-time PCR method in the heart tissue. The results of the present study showed that CK and LDH in MI were significantly higher in HIIT + MI (p < 0.05). myocardial infarction results in a significant increase in Drp1 gene expression in the MI and HIIT + MI groups relative to the Control group. The expression of the Drp1 gene was lower in the HIIT + MI group than in the MI group, but it was not statistically significant. Also, the results demonstrated that Mfn2 was no significant difference between the groups (p > 0.05). It seems that four weeks of exercise preconditioning with HIIT training reduced injury and necrosis in cardiac tissue and can increase cardio-protection. Also, no significant effect was observed in reducing Drp1 expression due to HIIT which may indicate the need for a longer training period.
Introduction
Acute Myocardial Infarction (AMI) occurs due to blockage of the cardiac vessels due to atherosclerotic plaque rupture, resulting in a decrease in oxygen and nutrients of the cardiac cells [1]. Myocardial infarction causes the death of cardiac cells through calcium overload, the opening of mitochondrial permeable transition pores (mPTP), and the production of mitochondrial oxidative stress, which is itself the result of mitochondrial dysfunction. Therefore, it seems that preventing the effects of MI and ischemia/reperfusion on mitochondrial function can be an important strategy for cardiac protection [2-4]. In fact, Mitochondrial fragmentation is associated with many diseases [5]. Mitochondria, a dynamic organelle, maintain their quality through the process of fusion (mixing of mitochondrial DNA, lipids, proteins, and metabolites) and fission (selective removal of damaged mitochondria by mitophagy), and these processes are essential for maintaining mitochondrial function [2]. Mitochondrial fusion mediating proteins are Mitofusions 1 and 2 (Mfn1, Mfn2) and Optic atrophy 1 (OPA1). While Dynamin-related peptide 1 (Drp 1) protein mediates mitochondrial fission through interaction with Fission protein 1 (Fis1 protein), mitochondrial fission factor (MFF), and Mitochondrial dynamics proteins of 49 and 51 kDa (MiD49, Mid51) [6]. During MI, mitochondrial homeostasis is disrupted, which includes its dynamics. In this regard, Jiang, et al. 2014 reported that after MI, mitochondrial dynamics change pathologically, which indicates a decrease in the expression of fusion proteins and an increase in fission proteins [7]. Various studies have also shown that Ischemia-reperfusion damage increases Drp1-dependent mitochondrial fission. However, more research is still needed on mitochondrial fusion [2,5,8-10]. Impaired mitochondrial fission leads to increased free radical production, disruption of calcium homeostasis, low Adenosine Triphosphate (ATP) production decreased energy metabolism, and overall mitochondrial dysfunction in mammalian cells [11-13]. Inhibition of Drp1 / Fis1 interaction following MI has been shown to reduce mitochondrial fragmentation and Reactive Oxygen Species (ROS) production and improve mitochondrial membrane potential and mitochondrial integrity [14]. In vitro and ex vivo studies, inhibition of mitochondrial fission in myocardial I / R injury, reduced myocardial infarct size, prevented cardiomyocyte apoptosis, and improved cardiac function [10]. On the other hand, increased expression of mitochondrial fusion proteins increases I / R damage resistance, so that cell death following ischemic injury is reduced. However, Mfn1 and Mfn2 cell defects in laboratory studies have reduced membrane potential [15]. Regarding cardiac protection strategies, epidemiological studies show a strong relationship between exercise and rescuing patients from MI. These studies have shown that the onset of ischemia-reperfusion injury (I / R) and MI is delayed by exercise. Exercise activity not only reduces cardiovascular risk factors but also enhances cardiac protection against ischemia-reperfusion [16]. Exercise by short-term ischemia exerts its effect as a pre-conditioning [17-20]. The effect of exercise pre-conditioning is such that it can provide cardiac protection from one session to a short time period of exercise training [20-23]. However, the effect of exercise pre-conditioning also depends on the model, volume, and especially the intensity of exercise and ischemia caused by exercise. In this regard, Luan, et al. 2019. Reached results in studying the beneficial effects of different types of exercise on 26 types of chronic diseases such as cardiovascular disease. According to the results of High-Intensity Interval Training (HIIT) compared to other exercises, it significantly improves cardiac function [24,25].
Exercise seems to activate protein kinases such as Calmodulin Kinase (CaMKs), AMPK, Mitogen-activated protein kinase p38 (p38MAPK), and Extracellular signal-Regulated Kinase (ERK) by increasing ROS, free phosphates, calcium ion levels, Adenosine Diphosphate (ADP), Adenosine Monophosphate (AMP) which resulting in mitochondrial dynamic regulation [26]. However, the exact cellular molecular mechanism of cardiac protection of exercise activity, especially High-intensity interval training, is still unclear. One of the protective pathways of the heart against ischemia seems to be to protect mitochondrial function, and the role of exercise pre-conditioning in this mechanism is worth studying [10]. Given the important role of changes in the structure and function of mitochondria that cause damage and death of cardiac cells during MI and I / R and also a positive effect of exercise training on genes and proteins associated with mitochondrial dynamics [27], the question and hypothesis arise whether a short-term period of 4 weeks of high-intensity interval training can reduce the damage caused by MI by having a positive effect on mitochondrial fission and fusion factors?
Materials and methods
Experimental design and treatment schedule
The present study is an experimental study that was performed with a post-test design to measure the main variables of the study (Drp1, Mfn2 expression, infarct size). Before the start of the research period, the research plan was presented to the ethics committee of the Sports Sciences Research Institute, and the ethics license was received with the code (IR.SSRC.REC.1400.017). After that, 24 adult male rats aged 16 weeks - 20 weeks with a weight range of 240 - 320 g were prepared as a sample from the Laboratory Animal Center of the Pasteur Institute and were transferred to the animal laboratory of the Faculty of Sports Sciences of Shahid Rajaei Teacher Training University. In the laboratory pet house according to the instructions of the Iranian Association for the Protection of Laboratory Animals and in standard conditions (temperature 22 ± 2 °C, relative humidity 55%) was maintained, and with the dark cycle of light 12:12, free access to water and food (pellets produced by Behparvar Company) and kept in clean cages with 3 rats in each cage. After a week of adaptation to the environment to familiarize the rats with the training environment (Iranian treadmill, made by Iranian Danesh Salar Company), rats ran on the treadmill for one week, every day with an intensity of 5 - 10 meters per minute for 10 to 15 minutes.
After the adjustment period, rats were randomly divided into four equal groups training (HIIT) (N = 6), training and myocardial infarction group (HIIT + MI) (N = 6), control and myocardial infarction group (MI) (N = 6) and Control group (N = 6). All research steps are summarized in Table 1.
V peak test
24 hours after the adjustment period and 48 hours before the start of the training period, the endurance capacity test and V peak estimation were performed based on the protocols used in previous studies. For measuring the V peak, rats first warmed up at a low speed of 10 meters per minute. Then, the experiment started by running rats at a speed of 15 meters per minute for two minutes. The treadmill speed was then automatically increased by 0.03 m / s (every 1.8 to 2 m / s) every two minutes until the rats were no longer able to continue running on the treadmill [28,29]. Exhaustion time was determined by mild shock. When rats struck the shock device twice at the end of the treadmill in 30 seconds or showed reflection and stood upright on their feet, they were considered exhausted [30]. This test was performed 24 hours after the end of the training period, to testify to the effect of training on the aerobic fitness of rats.
Training protocol
The training period, which lasted for 4 weeks, included: 5 consecutive days of HIIT training per week. Each session was warmed up for 5 minutes at a speed of 10 meters per minute, followed by 10 intervals of 1 minute of running on a treadmill.
In the first week, the intensity of rat exercise in training intervals was 85% V peak, in the second week, the intensity was 90% V peak, in the third week, the intensity was 95% V peak, and finally, in the fourth week, rats ran 100% V peak on the treadmill. Rats rested for 2 minutes between training intervals and cooled for 5 minutes at a speed of 6 m / min at the end of each training session. Training intensity was designed based on the amount of V peak. The Control group and the control group did not exercise on the treadmill during the training period. However, they were placed on the immobile treadmill to provide the same condition for all rats (Table 2).
Method of induction of myocardial infarction
Forty-eight hours after the V peak estimation test at the end of the training period, Isoproterenol (ISP) was prepared in a normal saline solution. This substance was injected subcutaneously (150 mg/kg body weight) on two consecutive days 24 hours apart to induce myocardial infarction [31,32] in MI and HIIT + MI groups. The use of this substance in animal models, especially rats, is one of the common methods for causing myocardial infarction. The Control group was injected to control the effect of the placebo injection.
Biopsy
Forty-eight hours after the second isoproterenol injection, rats were deeply anesthetized by intraperitoneal injection of Ketamine (100 mg/kg body weight) and Xylazine (10 mg/kg body weight), and the animal’s chest was dissected. Blood samples were taken directly from rat hearts and collected in Falcon tubes. After blood sampling from the heart, cardiectomy (removal of the heart from the body) was performed under sterile conditions. The isolated cardiac tissue was washed with Phosphate-Buffered Saline (PBS) and part of the cardiac tissue was placed in containers containing 4% formalin solution for staining. And part was placed in containers containing 20% RNAlater solution for gene expression and transferred to the laboratory for further experiments. Blood taken from the heart was poured into a CBC tube containing the EDTA anticoagulant type K2 (the tubes were gently shaken to completely dissolve the material in the blood) to collect serum. The serum of blood samples was isolated by SIGMA 2 - 15PK centrifugation at 3100 rpm and 15 °C for 15 minutes to measure cardiac injury markers. Tissue samples were then stored in the freezer at minus 70 and blood samples at minus 20 until further testing.
Confirmation of infarction
To stabilize cardiac tissue injury, blood levels of CK and LDH (markers indicating cardiomyocyte necrosis) were measured by using the standard enzymatic method of the German Societies for Clinical Chemistry (DGKC) ((CK: lot 1661120), (LDH: 1820721), Delta Darman Part, Iran) and activities of enzymes were expressed in mg/dl and U/l, respectively. All procedures were performed according to the kit’s instructions. Hematoxylin-Eosin staining (H&E) was used to evaluate necrotic damage.
Gene expression
A real-time polymerase chain reaction (RT-PCR method) was used to measure the gene expression of Mfn2 and Drp1 genes which includes the following steps: 1. primers were designed and synthesis based on: sequence of DNA from NCBI (The National Center for Biotechnology Information) database using the Oligo Analyzer 1.0.2 tool, also, having at least %GC, PCR product less than 220 pb, appropriate BLAST results and high specificity of 85% (Takapouzist Co, Iran), 2. Ribonucleic Acid (RNA) was prepared by homogenization of heart tissue and extraction with the total RNA extraction kit (Qiagen, German, Cat no: 74004), 3. the 260/280 nm absorbance ratio was used to determine the quality of RNA samples in a NanoDrop 2000c(r) Spectrophotometer (Thermo Scientific, Wilmington, DE, EUA), 4. synthesis of complementary DNA (Thermo Fisher Scientific, USA, Cat no: k-1622) according to the manufacturer’s guidelines, 5. amplification and detection by RT-PCR device (Corbbet, Qiagen, German) by Rotor-Gene 6000. SYBR® Green RT-PCR Master Mix (Parstous, Iran) by a thermal program as follows: One cycle at 94 °C for 10 min, 40 cycles at 95 °C for 15 s, 55 °C for 30 s, 72 ˚C for 45 s and 72 ˚C for 5 min. Glyceraldehyde-3-phosphate dehydrogenase was used as a housekeeping gene for mRNA analysis. Table 3 demonstrates the primer sequences. The logarithmic fold change (Log FC) was used to normalize fold changes and analyze gene expression data.
Statistical analysis
In this study, descriptive statistics were used to determine the mean and standard deviation. Shapiro-Wilk test was used to determine the normality of the data and one-way variance analysis and post hoc Bonferroni test were used to determine the differences between the groups. The significance level was considered p < 0.05. All data were also analyzed by SPSS software version 23.
Results
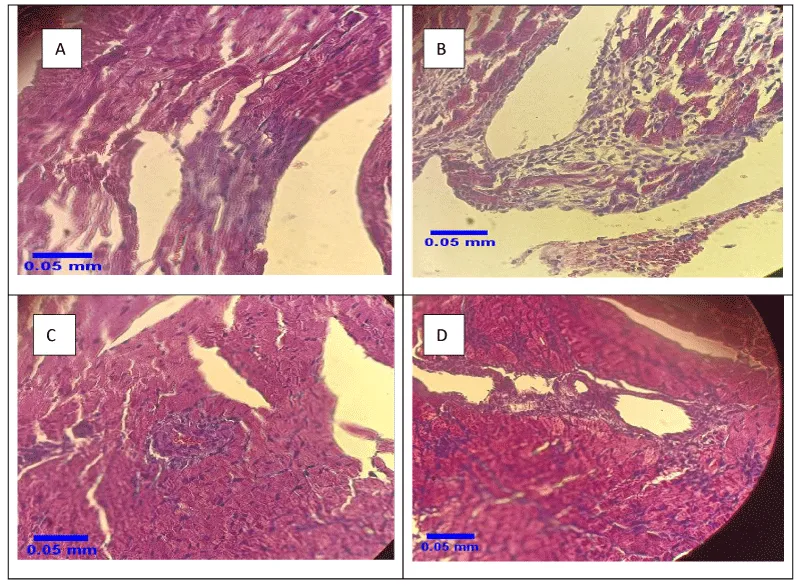
After staining, the samples were investigated using a light microscope with a magnification of × 40 (Figure 1). Severe (Figure 1A) and moderate (Figure 1B) MI were observed in two groups of MI and HIIT + MI, respectively.
Based on the results of the ANOVA test, there was no significant difference between the groups in weight (p = 0.71, F = 0.42) and V peak (p = 0.90, F = 0.16) at the beginning of the training period (p > 0.05). The characteristics of rats (weight and V peak) before and after training are given in Table 4.
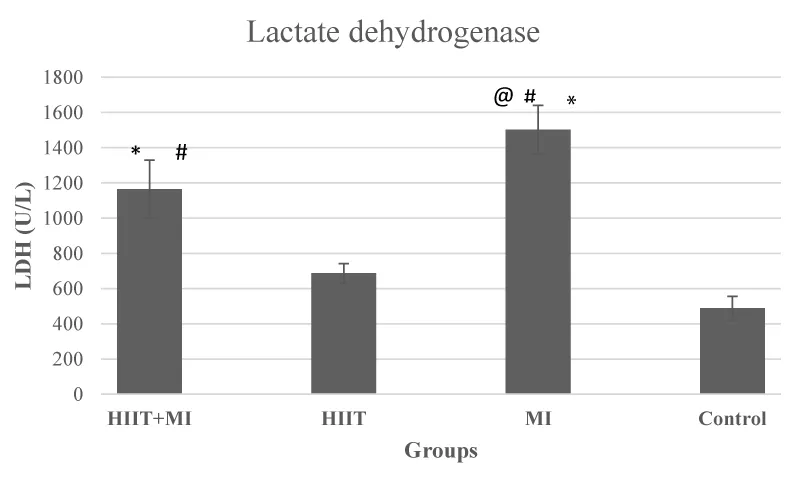
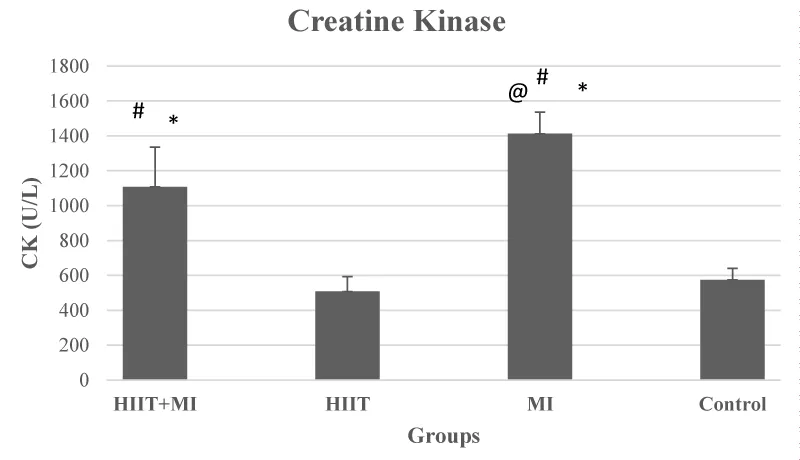
The results of the ANOVA test about the levels of blood CK and LDH showed that there was a significant difference between the study groups (F values were 26.50 and 34.15, respectively) and p < 0.001. Also, based on the results of the post hoc test, CK and LDH levels in HIIT + MI and MI groups were significantly different from the Control group and HIIT group (p < 0.05). CK and LDH levels in the HIIT + MI group were significantly reduced compared to the MI group (p < 0.05) (Figures 2 and 3).
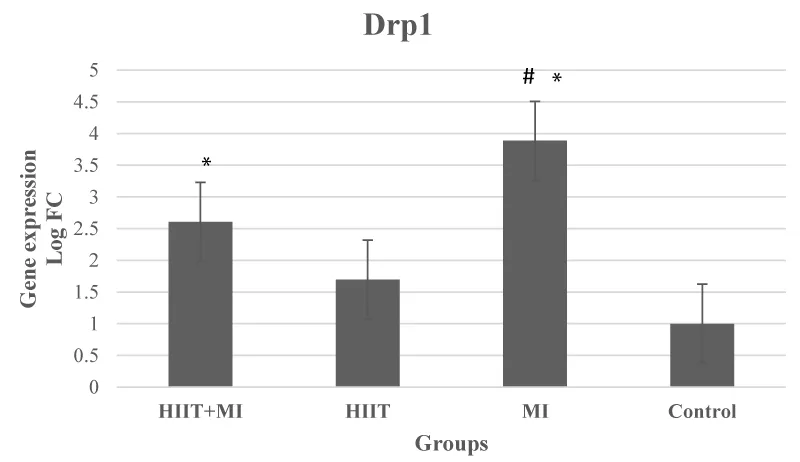
Based on the results of data analysis, the expression of the Drp1 gene in cardiac tissue after 4 weeks of HIIT and induction of infarction was significantly different between groups (p = 000, F = 17.71) (p < 0.05). According to the Bonferroni test, Drp1 had a significant increase in MI and HIIT + MI groups compared to the Control group (p < 0.001). Also, the increase in the expression of this gene was significant in the MI group compared to the HIIT group (p < 0.001). Drp1 gene expression was increased in the MI group compared to HIIT + MI but this increase was not significant (p > 0.05) (Figure 4).
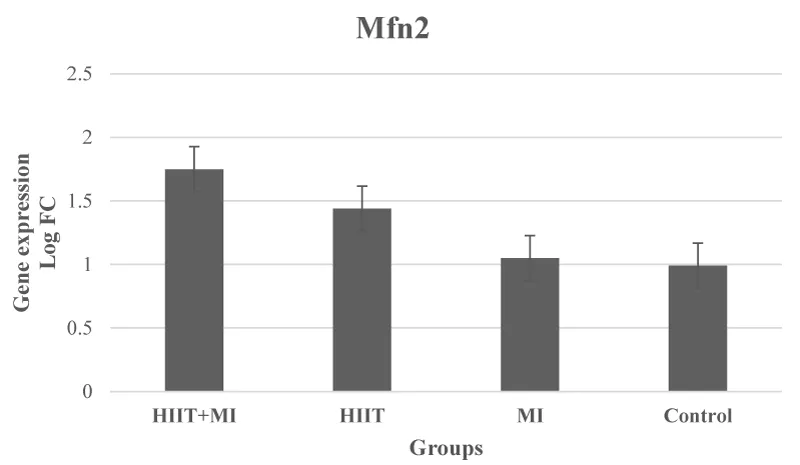
The results of One-way ANOVA in relation to Mfn2 gene levels did not show a significant difference between the groups (p = 0.19, F = 1.74) (Figure 5).
Discussion
The number of cardiac protection strategies is increasing, and all of these strategies try to reduce the amount of tissue damage following ischemia caused by changes in myocardial blood flow and the loss of fewer cardiac cells. The major protection strategies studied include ischemic pre-conditioning, ischemic post-conditioning, heat stress, oxidative stress, and certain drug interventions [33]. However, different methods have been identified to achieve cardiac protection against I / R damage, However, several studies have concluded that one of the best most effective, and tolerable approaches to achieve cardiac protection against IR cardiac damage is regular exercise training [33,34]. Therefore, this study aimed to investigate the effect of exercise preconditioning with 4 weeks of HIIT training on cardiac damage and mitochondrial dynamic proteins as effective factors of exercise in cardiac protection following MI. As you can see in Figure 1, the pathological results of this study showed that induction of myocardial infarction with isoproterenol, a beta-adrenoceptor agonist, led to necrosis of heart tissue. This injury was very severe in the MI group. Moderate necrosis was observed in the HIIT + MI group. This finding was in line with the results of studies such as Ghanimati, et al. 2020 [30] and Shukla, et al. 2015 [32]. An increase in blood markers of tissue injury (creatine kinase and lactate dehydrogenase) was also observed in the MI and HIIT + MI groups in this study, which indicates the severity of MI damage. LDH is a blood indicator of cardiac tissue injury that is secreted with a delay and peaks two to three days after MI. While creatine kinase is secreted immediately after MI [32]. In our study, sampling was performed 48 hours after the last isoproterenol injection. The high level of these factors in the MI group compared to the other groups was in line with the pathology results. Also, low blood levels of these factors in the HIIT + MI group compared to the MI group show the effect of cardiac protection of exercise, which has been reported in previous studies [25,30,35]. In various studies, increasing glycolytic flow, improving nitric oxide signals, increasing the production of Heat Shock Proteins (HSPs), increasing the function of sarcolemma or mitochondrial ATP-sensitive potassium channels, increasing the antioxidant capacity of cytosol, reducing ROS, altering synthesis and mitochondrial phenotypes are some of the cellular mechanisms reported by exercise-induced cardiac protection [16,27]. Studies also point to the role of Akt, PGC1α activation, and mitochondrial biogenesis due to exercise activity in reducing heart damage [36].
Based on the findings of the present study, myocardial infarction led to a significant increase in Drp1 gene expression in the MI and HIIT + MI groups compared to the Control group, which has been observed in other studies [15,37]. Although the expression of the Drp1 gene in the HIIT + MI group was lower than the MI group, it was not significant. Accumulation of intracellular calcium during severe myocardial ischemia appears to activate calcineurin, which dephosphorylates Drp1 in Ser637 and increases fission [1]. On the other hand, it has been suggested that exercise promotes improved mitochondrial function by improving biogenesis, fission, fusion, and mitophagy, formation of new mitochondria, and identification and removal of damaged and dysfunctional mitochondria [38]. In this regard, Jiang, et al. 2014 showed a decrease in Drp1 after eight weeks of aerobic interval training on a treadmill [7]. Decreased Drp1 has also been observed after 5 days of treadmill running training [39]. However, running a marathon (24 hours) resulted in higher Drp1 activity in young athletes [40]. Some studies did not find any change in Drp1 levels due to exercise [41,42]. In our study, Drp1 did not change in the HIIT group compared to the Control group. However, exercise through protein kinase A and AMPK appears to increase Drp1 phosphorylation in Ser637, inhibit the interaction between Drp1-Fis1, and inhibit Drp1 phosphorylation in Ser616 thereby reducing mitochondrial fission [43,44]. Also, according to the results of this study, despite the increase in Mfn2 in the HIIT and HIIT + MI groups, this increase was not significant compared to the Control group. The MI group did not show a significant difference compared to the Control group. Increases in Mfn1 and Fis1 proteins have been reported in the muscles of healthy young individuals following intensity interval training [45]. Also, an increase in mitochondrial fusion proteins was observed in rats after 7 days of chronic muscle contraction 3 hours a day at 10 Hz (endurance training model). 7 days of muscle denervation reduced the levels of this protein [46]. However, some animal studies on the effect of exercise on fusion proteins [7,22,39,41,42,47] have reported different results such as reduction and no change, which seem to be different results depending a lot on the intensity and duration of the training protocol. Taken together, these findings suggest that exercise positively regulates fission and fusion processes, and that type of activity plays an important role in the mitochondrial dynamic response [26,48]. Also, a decrease in the size of myocardial infarction has been shown by increasing the expression of Mfn1 / 2 and decreasing Drp1 due to exercise training [37]. PGC1α also regulates the translation of Mfn1 and Mfn2 based on the findings of laboratory studies [38]. The effect of four weeks of exercise training on PGC1α increase has already been reported, although we did not measure this factor [22,49].
Conclusion
Although extrapolation of findings from experimental animals to humans should be done cautiously, the results of the present study suggest that exercise preconditioning with HIIT even for short term (4 weeks) reduced injury and necrosis in cardiac tissue. further research is needed to show regular exercise especially HIIT can increase cardio-protection by improvement of mitochondrial dynamic. It also seems that a longer period of exercise training is needed to prove the effect of mitochondrial dynamics on cardiac protection.
Funding statement
In this paper, the authors did not receive funding from any institution or company and declared that they do not have any conflict of interest.
- Ramachandra CJA, Hernandez-Resendiz S, Crespo-Avilan GE, Lin YH, Hausenloy DJ. Mitochondria in acute myocardial infarction and cardioprotection. EBioMedicine. 2020 Jul;57:102884. doi: 10.1016/j.ebiom.2020.102884. Epub 2020 Jul 10. PMID: 32653860; PMCID: PMC7355051.
- Hall AR, Burke N, Dongworth RK, Kalkhoran SB, Dyson A, Vicencio JM, Dorn GW II, Yellon DM, Hausenloy DJ. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 2016 May 26;7(5):e2238. doi: 10.1038/cddis.2016.139. Erratum in: Cell Death Dis. 2021 Jun 30;12(7):660. PMID: 27228353; PMCID: PMC4917668.
- Ježek J, Cooper KF, Strich R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants (Basel). 2018 Jan 16;7(1):13. doi: 10.3390/antiox7010013. PMID: 29337889; PMCID: PMC5789323.
- Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants (Basel). 2019 Oct 6;8(10):454. doi: 10.3390/antiox8100454. PMID: 31590423; PMCID: PMC6826663.
- Zhou Z, Wang Z, Guan Q, Qiu F, Li Y, Liu Z, Zhang H, Dong H, Zhang Z. PEDF Inhibits the Activation of NLRP3 Inflammasome in Hypoxia Cardiomyocytes through PEDF Receptor/Phospholipase A2. Int J Mol Sci. 2016 Dec 12;17(12):2064. doi: 10.3390/ijms17122064. PMID: 27973457; PMCID: PMC5187864.
- Trewin AJ, Berry BJ, Wojtovich AP. Exercise and Mitochondrial Dynamics: Keeping in Shape with ROS and AMPK. Antioxidants (Basel). 2018 Jan 6;7(1):7. doi: 10.3390/antiox7010007. PMID: 29316654; PMCID: PMC5789317.
- Jiang HK, Wang YH, Sun L, He X, Zhao M, Feng ZH, Yu XJ, Zang WJ. Aerobic interval training attenuates mitochondrial dysfunction in rats post-myocardial infarction: roles of mitochondrial network dynamics. Int J Mol Sci. 2014 Mar 26;15(4):5304-22. doi: 10.3390/ijms15045304. PMID: 24675698; PMCID: PMC4013565.
- Cooper HA, Eguchi S. Inhibition of mitochondrial fission as a novel therapeutic strategy to reduce mortality upon myocardial infarction. Clin Sci (Lond). 2018 Oct 18;132(20):2163-2167. doi: 10.1042/CS20180671. PMID: 30341226.
- Javadov S, Rajapurohitam V, Kilić A, Hunter JC, Zeidan A, Said Faruq N, Escobales N, Karmazyn M. Expression of mitochondrial fusion-fission proteins during post-infarction remodeling: the effect of NHE-1 inhibition. Basic Res Cardiol. 2011 Jan;106(1):99-109. doi: 10.1007/s00395-010-0122-3. Epub 2010 Oct 1. PMID: 20886221.
- Maneechote C, Palee S, Chattipakorn SC, Chattipakorn N. Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med. 2017 Nov;21(11):2643-2653. doi: 10.1111/jcmm.13330. Epub 2017 Sep 22. PMID: 28941171; PMCID: PMC5661112.
- Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012 Jan;32(2):309-19. doi: 10.1128/MCB.05603-11. Epub 2011 Nov 14. PMID: 22083962; PMCID: PMC3255771.
- Reddy PH. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J Alzheimers Dis. 2014;40(2):245-56. doi: 10.3233/JAD-132060. PMID: 24413616; PMCID: PMC3972337.
- Zhou K, Shi L, Wang Z, Zhou J, Manaenko A, Reis C, Chen S, Zhang J. RIP1-RIP3-DRP1 pathway regulates NLRP3 inflammasome activation following subarachnoid hemorrhage. Exp Neurol. 2017 Sep;295:116-124. doi: 10.1016/j.expneurol.2017.06.003. Epub 2017 Jun 2. PMID: 28579326.
- Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013 Oct 8;2(5):e000461. doi: 10.1161/JAHA.113.000461. PMID: 24103571; PMCID: PMC3835263.
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010 May 11;121(18):2012-22. doi: 10.1161/CIRCULATIONAHA.109.906610. Epub 2010 Apr 26. PMID: 20421521.
- Borges JP, Lessa MA. Mechanisms Involved in Exercise-Induced Cardioprotection: A Systematic Review. Arq Bras Cardiol. 2015 Jul;105(1):71-81. doi: 10.5935/abc.20150024. Epub 2015 Mar 27. PMID: 25830711; PMCID: PMC4523290.
- Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H19-27. doi: 10.1152/ajpheart.00712.2006. Epub 2006 Sep 8. PMID: 16963615; PMCID: PMC3242363.
- Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J Appl Physiol (1985). 2011 Sep;111(3):905-15. doi: 10.1152/japplphysiol.00004.2011. Epub 2011 Mar 10. PMID: 21393468.
- Quindry JC, Miller L, McGinnis G, Kliszczewicz B, Irwin JM, Landram M, Urbiztondo Z, Nanayakkara G, Amin R. Ischemia reperfusion injury, KATP channels, and exercise-induced cardioprotection against apoptosis. J Appl Physiol (1985). 2012 Aug;113(3):498-506. doi: 10.1152/japplphysiol.00957.2011. Epub 2012 May 31. PMID: 22653992; PMCID: PMC3426170.
- Thijssen DHJ, Redington A, George KP, Hopman MTE, Jones H. Association of Exercise Preconditioning With Immediate Cardioprotection: A Review. JAMA Cardiol. 2018 Feb 1;3(2):169-176. doi: 10.1001/jamacardio.2017.4495. PMID: 29188285.
- Shen YJ, Pan SS, Zhuang T, Wang FJ. Exercise preconditioning initiates late cardioprotection against isoproterenol-induced myocardial injury in rats independent of protein kinase C. J Physiol Sci. 2011 Jan;61(1):13-21. doi: 10.1007/s12576-010-0116-9. Epub 2010 Oct 13. PMID: 20941560.
- Tao L, Bei Y, Lin S, Zhang H, Zhou Y, Jiang J, Chen P, Shen S, Xiao J, Li X. Exercise Training Protects Against Acute Myocardial Infarction via Improving Myocardial Energy Metabolism and Mitochondrial Biogenesis. Cell Physiol Biochem. 2015;37(1):162-75. doi: 10.1159/000430342. Epub 2015 Aug 17. PMID: 26303678.
- Wu NN, Tian H, Chen P, Wang D, Ren J, Zhang Y. Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health. Cells. 2019 Nov 14;8(11):1436. doi: 10.3390/cells8111436. PMID: 31739509; PMCID: PMC6912418.
- Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, Wang R. Exercise as a prescription for patients with various diseases. J Sport Health Sci. 2019 Sep;8(5):422-441. doi: 10.1016/j.jshs.2019.04.002. Epub 2019 Apr 18. PMID: 31534817; PMCID: PMC6742679.
- Rahimi M, Shekarforoush S, Asgari AR, Khoshbaten A, Rajabi H, Bazgir B, Mohammadi MT, Sobhani V, Shakibaee A. The effect of high intensity interval training on cardioprotection against ischemia-reperfusion injury in wistar rats. EXCLI J. 2015 Feb 20;14:237-46. doi: 10.17179/excli2014-587. PMID: 26417361; PMCID: PMC4555214.
- Ebadi B, Damirchi A, Alamdari KA, Darbandi-Azar A, Naderi N. Cardiomyocyte mitochondrial dynamics in health and disease and the role of exercise training: A brief review. Research in Cardiovascular Medicine. 2018;7(3):107.
- Powers SK, Smuder AJ, Kavazis AN, Quindry JC. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda). 2014 Jan;29(1):27-38. doi: 10.1152/physiol.00030.2013. PMID: 24382869; PMCID: PMC3929117.
- Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol Respir Environ Exerc Physiol. 1979 Dec;47(6):1278-83. doi: 10.1152/jappl.1979.47.6.1278. PMID: 536299.
- Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007 Dec;14(6):753-60. doi: 10.1097/HJR.0b013e3281eacef1. PMID: 18043295.
- Ghanimati R, Rajabi H, Ramezani F, Ramez M, Bapiran M, Nasirinezhad F. The effect of preconditioning with high-intensity training on tissue levels of G-CSF, its receptor and C-kit after an acute myocardial infarction in male rats. BMC Cardiovasc Disord. 2020 Feb 11;20(1):75. doi: 10.1186/s12872-020-01380-w. PMID: 32046645; PMCID: PMC7011373.
- Boarescu PM, Chirilă I, Bulboacă AE, Bocșan IC, Pop RM, Gheban D, Bolboacă SD. Effects of Curcumin Nanoparticles in Isoproterenol-Induced Myocardial Infarction. Oxid Med Cell Longev. 2019 May 7;2019:7847142. doi: 10.1155/2019/7847142. PMID: 31205590; PMCID: PMC6530192.
- Shukla SK, Sharma SB, Singh UR. β-Adrenoreceptor Agonist Isoproterenol Alters Oxidative Status, Inflammatory Signaling, Injury Markers and Apoptotic Cell Death in Myocardium of Rats. Indian J Clin Biochem. 2015 Jan;30(1):27-34. doi: 10.1007/s12291-013-0401-5. Epub 2014 Jan 23. PMID: 25646038; PMCID: PMC4310843.
- Kavazis AN. Exercise preconditioning of the myocardium. Sports Med. 2009;39(11):923-35. doi: 10.2165/11317870-000000000-00000. PMID: 19827860.
- Quindry J, French J, Hamilton K, Lee Y, Mehta JL, Powers S. Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp Gerontol. 2005 May;40(5):416-25. doi: 10.1016/j.exger.2005.03.010. Epub 2005 Apr 26. PMID: 15919594.
- Zhang KR, Liu HT, Zhang HF, Zhang QJ, Li QX, Yu QJ, Guo WY, Wang HC, Gao F. Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism. Apoptosis. 2007 Sep;12(9):1579-88. doi: 10.1007/s10495-007-0090-8. PMID: 17505785.
- Wang H, Bei Y, Lu Y, Sun W, Liu Q, Wang Y, Cao Y, Chen P, Xiao J, Kong X. Exercise Prevents Cardiac Injury and Improves Mitochondrial Biogenesis in Advanced Diabetic Cardiomyopathy with PGC-1α and Akt Activation. Cell Physiol Biochem. 2015;35(6):2159-68. doi: 10.1159/000374021. Epub 2015 Apr 7. PMID: 25896313.
- Ghahremani R, Damirchi A, Salehi I, Komaki A, Esposito F. Mitochondrial dynamics as an underlying mechanism involved in aerobic exercise training-induced cardioprotection against ischemia-reperfusion injury. Life Sci. 2018 Nov 15;213:102-108. doi: 10.1016/j.lfs.2018.10.035. Epub 2018 Oct 22. PMID: 30355530.
- Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012 Jul;40(3):159-64. doi: 10.1097/JES.0b013e3182575599. PMID: 22732425; PMCID: PMC3384482.
- Lee S, Kim M, Lim W, Kim T, Kang C. Strenuous exercise induces mitochondrial damage in skeletal muscle of old mice. Biochem Biophys Res Commun. 2015 May 29;461(2):354-60. doi: 10.1016/j.bbrc.2015.04.038. Epub 2015 Apr 15. PMID: 25887799.
- Jamart C, Francaux M, Millet GY, Deldicque L, Frère D, Féasson L. Modulation of autophagy and ubiquitin-proteasome pathways during ultra-endurance running. J Appl Physiol (1985). 2012 May;112(9):1529-37. doi: 10.1152/japplphysiol.00952.2011. Epub 2012 Feb 16. PMID: 22345427.
- Garnier A, Fortin D, Zoll J, N'Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005 Jan;19(1):43-52. doi: 10.1096/fj.04-2173com. PMID: 15629894.
- Kitaoka Y, Nakazato K, Ogasawara R. Combined effects of resistance training and calorie restriction on mitochondrial fusion and fission proteins in rat skeletal muscle. J Appl Physiol (1985). 2016 Sep 1;121(3):806-810. doi: 10.1152/japplphysiol.00465.2016. Epub 2016 Aug 18. PMID: 27539498.
- Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, Stöckli J, Burchfield JG, Jensen TE, Jothi R, Kiens B, Wojtaszewski JF, Richter EA, James DE. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015 Nov 3;22(5):922-35. doi: 10.1016/j.cmet.2015.09.001. Epub 2015 Oct 1. Erratum in: Cell Metab. 2015 Nov 3;22(5):948. PMID: 26437602; PMCID: PMC4635038.
- Tanaka T, Nishimura A, Nishiyama K, Goto T, Numaga-Tomita T, Nishida M. Mitochondrial dynamics in exercise physiology. Pflugers Arch. 2020 Feb;472(2):137-153. doi: 10.1007/s00424-019-02258-3. Epub 2019 Feb 1. PMID: 30707289.
- Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010 Dec 1;588(Pt 23):4795-810. doi: 10.1113/jphysiol.2010.199448. Epub 2010 Oct 4. PMID: 20921196; PMCID: PMC3010147.
- Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013 Dec;48(6):963-70. doi: 10.1002/mus.23838. PMID: 23494933.
- Picard M, Gentil BJ, McManus MJ, White K, St Louis K, Gartside SE, Wallace DC, Turnbull DM. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J Appl Physiol (1985). 2013 Nov;115(10):1562-71. doi: 10.1152/japplphysiol.00819.2013. Epub 2013 Aug 22. PMID: 23970537; PMCID: PMC3841825.
- Joseph AM, Adhihetty PJ, Leeuwenburgh C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J Physiol. 2016 Sep 15;594(18):5105-23. doi: 10.1113/JP270659. Epub 2015 Nov 21. PMID: 26503074; PMCID: PMC5023701.
- Hoshino D, Yoshida Y, Kitaoka Y, Hatta H, Bonen A. High-intensity interval training increases intrinsic rates of mitochondrial fatty acid oxidation in rat red and white skeletal muscle. Appl Physiol Nutr Metab. 2013 Mar;38(3):326-33. doi: 10.1139/apnm-2012-0257. Epub 2013 Mar 15. PMID: 23537026.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
