Annals of Molecular and Genetic Medicine
Micronutrient-Induced Activation of Nrf2 and -Elevation of Intracellular Antioxidants for Reducing Oxidative Stress and Chronic Inflammation in Diabetes
Kedar N Prasad*
Cite this as
Prasad KN (2017) Micronutrient-Induced Activation of Nrf2 and -Elevation of Intracellular Antioxidants for Reducing Oxidative Stress and Chronic Inflammation in Diabetes. Ann Mol Genet Med 1(1): 001-007. DOI: 10.17352/amgm.000001Despite extensive research and dietary and lifestyle recommendations, the incidence of diabetes continues to increase. Diabetic medications have been useful in controlling blood levels of glucose; however, in general, the disease continues to progress rather slowly, and eventually leads to diabetic-related complications. Analysis of published studies suggests that increased oxidative stress and chronic inflammation initiate and promote the progression of diabetes and diabetic–related complications. Classical anti-diabetic measures are aimed at control of glucose levels and do not reduce these factors. Therefore, together with standard care and adoption of appropriate diet and lifestyle, attenuation of these biochemical defects may have utility in the prevention, and in combination with standard care, for the improved management of diabetes. The question arises how to maximally and at the same time reduce oxidative stress and inflammation. This review describes how simultaneous attenuation of excess oxidative stress and inflammation may be enabled. This briefly discusses diverse actions and cellular distributions of different antioxidants in the body, and describes the effects of individual antioxidants in prevention and management of diabetes. It proposes that increasing the levels of antioxidant enzymes and dietary and endogenous antioxidant compounds at the same time is needed to decrease oxidative damage and chronic inflammation. An oral supplementation can increase the levels of antioxidant compounds; however, increasing the levels of antioxidant enzymes requires activation of Nrf2. This review discusses activation of Nrf2, and proposes a mixture of micronutrients that enhances the levels of antioxidant enzymes by activating the Nrf2 pathway, and also increases intracellular levels of antioxidant compounds. This combination is likely to have utility in patients with diabetes.
Introduction
Diabetes mellitus is characterized by hyperglycemia that results from insufficient or lack of production of insulin by the pancreas. Insulin resistance develops due to oxidative damage to the glucose transporter proteins or insulin receptors can lead to hyperglycemia. Diabetes has reached epidemic proportions throughout the world. In the USA, about 19 million people had diabetes in 2010; this number increased to 21 million in 2014. The number of undiagnosed case also rose from 7 million in 2010 to 8.1 million in 2014 (Center for Disease Prevention, 2014 National Diabetes Statistic report). The cost of diabetes and pre-diabetes care in the USA is $322.00 billion per year (American Diabetic Association, 2016). Despite extensive basic and clinical studies, the number of diabetes cases continues to increase. One of the reasons could be that the current recommendations, such as, losing weight, doing daily moderate exercise, eating a balanced diet, and stopping tobacco smoking are not being followed by most people.
The role of reactive oxygen species (ROS) in normal cell functions is complex depending upon their levels in the cells. Low levels of ROS activate cell-signaling pathways that are essential for initiating biochemical and physiological processes necessary for the survival and functions of the cells. A review has discussed these issues in detail [1]. On the other hand, high levels of ROS referred as oxidative stress induces cellular damage including DNA, RNA protein and membranes. Analysis of published studies has concluded that increased oxidative stress and chronic inflammation are primarily responsible for the initiation and progression of diabetes as well as for the development of diabetes-related complications [2]. Since then, several studies confirming the role of oxidative stress in diabetes have also been published [3-10]. Increased oxidative damage, if not repaired, can lead to chronic inflammation that releases free radicals, pro-inflammatory cytokines, complement proteins, adhesion molecules and prostaglandins, all of which are toxic to cells. This issue has been discussed in a previous review [2]. Since then, additional studies on the role of chronic inflammation in diabetes and diabetic-related complications have been published [11-18]. Strongest support for the role of oxidative stress comes from the report that markers of oxidative damage were elevated in pre-diabetic patients [6], and in the parents of diabetic as well as children with type 1 diabetes [19,20].
Increased oxidative stress and chronic inflammation can damage the pancreas, glucose transporter proteins and insulin receptors that contribute to hyperglycemia. If the pancreas is producing sufficient amounts of insulin, oxidative damage to the glucose transporter proteins or insulin receptors may cause tissue resistance to insulin leading to hyperglycemia. Since these biochemical defects initiate and promote diabetes, reducing these biochemical defects may be one of the rational choices for the prevention, and in combination with standard treatment, for the improved management of diabetes.
Despite advances in medications for controlling blood glucose levels, the disease slowly continues to progress. This may in part be because these medications do not adequately address the issues of increased oxidative stress and chronic inflammation.
This review briefly discusses diverse actions and cellular distributions of different antioxidants in the body, and describes the effects of individual antioxidants in prevention and management of diabetes. It proposes that increasing the levels of antioxidant enzymes and dietary and endogenous antioxidant compounds at the same time is needed to decrease oxidative stress and chronic inflammation. An oral supplementation can increase the levels of antioxidant compounds; however, increasing the levels of antioxidant enzymes requires activation of Nrf2. This review discusses how an appropriate mixture of micronutrients can simultaneously enable activation of Nrf2 pathway, leading to elevation of antioxidant enzymes, and directly enhance levels of intracellular antioxidants.
Diverse actions and cellular distributions of antioxidants in the body
The body protects against oxidative damage by antioxidant compounds derived from the diet as well as made in the body, and antioxidant enzymes made in the body. Antioxidant compounds reduce oxidative stress by a mechanism in part different from that of antioxidant enzymes. They destroy free radicals by donating electron to unpaired state of the free radicals. On the other hand, antioxidant enzymes destroy free radicals by catalysis (converting them to the harmless molecules such as to water and oxygen). Some antioxidant compounds also reduce chronic inflammation [21-25]. Different antioxidants have different affinities for free radicals depending upon their location in the cellular compartments. Water-soluble antioxidants such as vitamin C and glutathione protect molecules in the aqueous environment of the cells, whereas lipid soluble antioxidants such as vitamin A and vitamin E protect molecules in the lipid environment of the cells. Vitamin E was more effective in quenching free radicals in a reduced oxygenated cellular environment, whereas vitamin C and vitamin A were more effective in a higher oxygenated environment of the cells [26]. Vitamin C is important for recycling the oxidized form of vitamin E to the antioxidant form [27]. In addition, antioxidants produce cell protective proteins by upregulating or downregulating different microRNAs [28]. For example, some antioxidant can activate Nrf2 by upregulating miR-200a that inhibits its target protein Keap1 [29], whereas others activate Nrf2 by downregulating miR-21 that binds with 3-UTR Nrf2 mRNA [29].
Effects a single antioxidant compound on type 2 diabetes
Because of the complexity and different mechanisms of action and cellular distributions of different antioxidants, it is not possible to simultaneously reduce oxidative stress and inflammation by a single antioxidant or anti-inflammatory agent. Nevertheless, previous studies have investigated the effects of using a single antioxidant primarily in animal models of diabetes. These antioxidants include vitamin A, vitamin C, vitamin D, vitamin E, alpha-lipoid acid, n-acetylcysteine, L-carnitine, coenzyme Q10, folic acid, thymine, omega-3-fatty acids, and chromium. The studies on the effects of these individual antioxidants in diabetes have been previously reviewed [2]. Treatment with a single antioxidant was found to lead to some beneficial effects in animal models and in patients with diabetes. Since then, additional studies on the effects of a single antioxidant, such as sulphoraphane, riboflavin, curcumin, resveratrol, alpha-lipoic acid, vitamin C, omega-3-fatty acids, and coenzyme Q10 on animal models of diabetes and human diabetes have produced similar results [30-37]. In some studies supplementation with resveratrol had no effect on glycemic control [38], selenium had no effect on insulin resistance [39], and chromium compound had no effect on glycated hemoglobin (A1C) in patients with type 2 diabetes [40]. One of the reasons for the inconsistent benefits with a single antioxidant in patients with diabetes could be that a single antioxidant failed to simultaneously reduce oxidative stress inflammation in these patients. Other reasons could be that a single antioxidant in high oxidative environment of diabetic patients is oxidized and then acts as a pro-oxidant rather than as an antioxidant.
Based on the differential actions and distributions of antioxidant enzymes and antioxidant compounds, simultaneous increase in the levels of antioxidant enzymes through the activation of Nrf2/ARE (nuclear transcriptional factor-2/antioxidant response element) pathway, together with administration of dietary and endogenous antioxidant compounds may be necessary for optimally reducing oxidative stress and chronic inflammation in Alzheimer’s disease [41]. A similar strategy may be needed for reducing these biochemical defects in patients with diabetes. The levels of antioxidant compounds can easily be enhanced by an oral supplementation; however, increasing the levels of antioxidant enzymes is complex requiring an activation of the Nrf2/ARE pathway.
Nuclear transcriptional factor-2 (Nrf2)
The Nrf2 (nuclear factor-erythroid-2- related factor 2) belongs to the Cap ´N´Collar (CNC) family that contains a conserved basic leucine zipper (bZIP) transcriptional factor [42]. Under physiological condition, Nrf2 is associated with Kelch-like ECH associated protein 1 (Keap1) that acts as an inhibitor of Nrf2 [43]. Keap1 protein serves as an adaptor to link Nrf2 to the ubiquitin ligase CuI-Rbx1 complex for degradation by proteasomes and maintains the steady levels of Nrf2 in the cytoplasm. Nrf2-keap1 complex is primarily located in the cytoplasm. Keap1 acts as a sensor for ROS/electrophilic stress.
Activation of Nrf2
Activation of Nrf2 during acute oxidative stress: Acute oxidative stress occurs during aerobic exercise, treatment of cells with chemicals such as H2O2 for a short period of time [44], and treatment of animals with the donors of free radicals, such as 3,3’5-triiodo-l-thyronine, a thyroid hormone for a few hours [45]. During acute oxidative stress, ROS (reactive oxygen species) activates Nrf2 which then dissociates itself from Keap1- CuI-Rbx1 complex and translocates in the nucleus where it heterodimerizes with a small Maf protein and binds with ARE (antioxidant response element) leading to increased expression of target genes coding for several cytoprotective enzymes including antioxidant enzymes and phase-2-detoxifying enzymes [46-48]. Activation of Nrf2 by a ROS-dependent mechanism is effective in reducing oxidative damage only under acute oxidative stress.
Activation of Nrf2 during chronic oxidative stress: In contrast to acute oxidative stress, Nrf2 becomes resistant to ROS during chronic oxidative stress in neurodegenerative disease [49-51], suggesting that activation of Nrf2 by a ROS-independent mechanism exists. This is also confirmed in patients with diabetes who show increased oxidative stress causing cellular damage continues to occur despite the presence of Nrf2 [2]. The issue then is how to activate ROS-resistant Nrf2 in patients with diabetes.
Antioxidants regulating activation of Nrf2 during acute oxidative stress
ROS generated during acute oxidative stress are essential for activating Nrf2. Pre- treatment with antioxidants such as NAC (n-acetylcysteine) during acute oxidative stress prevented activation of Nrf2 [44,45]. It appears that pretreatment with an antioxidant prevented oxidative damage by scavenging all ROS. Since ROS was not available, Nrf2 was not activated. These results should not be interpreted to mean that lack of activation of Nrf2 in the presence of an antioxidant is harmful. In the studies described above, NAC directly substituted for Ntf2 by removing free radicals during acute oxidative stress.
Antioxidants regulating activation of Nrf2 during chronic oxidative stress
Antioxidant compounds activate Nrf2 during chronic stress without the need for ROS.
Some examples of such antioxidant compounds include vitamin E and genistein [52], alpha-lipoic acid [53], curcumin [54], resveratrol [55,56], omega-3-fatty acids [57,58], glutathione [59], NAC [60], and coenzyme Q10 [61]. Several plant-derived phytochemicals, such as epigallocatechin-3-gallate, carestol, kahweol, cinnamonyl-based compounds, zerumbone, lycopene and carnosol [42,62,63], genistein [52], allicin, a major organosulfur compound found in garlic [64], sulforaphane, a organosulfur compound, found in cruciferous vegetables [65], and kavalactones (methysticin, kavain and yangonin) [66]. The exact reasons for the activation of Nrf2 by antioxidant compounds in the absence of ROS are unclear. Analysis of previous studies suggests that antioxidants may activate Nrf2 by altering the expression levels of microRNAs [28].
Regulation of activation of Nrf2 by MicroRNAs
MicroRNAs) are evolutionary conserved small non-coding endogenous single-stranded RNAs of approximately 22 nucleotides in length, and are present in all living organisms including humans [67-70]. Each miRNA binds with the 3’-UTR (3’-untranslated region) of a specific mRNA causing its degradation, thereby, reducing the formation of its target protein [69]. The role of microRNAs in regulating the activation of Nrf2 in diabetes is briefly described here.
Diabetic mice with nephropathy had reduced expression levels of miR-200a and increased expression of miR-21 [29]. MiR-21 binds with the 3’-UTR mRNA of Nrf2; leading to decreased expression of Nrf2. Thus reduced expression of miR-21 would increase the levels of Nrf2. Treatment of mice with curcumin analog C66 that exhibits antioxidant and anti-inflammation activities increased the expression of renal miR-200a that inhibited Keap1, an inhibitor of Nrf2, which allows activation of Nrf2. Treatment of mice with the curcumin analog C66 reduced the expression of miR21 that increases the activation of Nrf2. These results show that increased expression levels of miR-200a enhances Nrf2 activation by inhibiting Keap1 levels, whereas decreased expression levels of miR-21 increases the levels of Nrf2 by reducing its binding to the Nrf2 mRNA [29].
Binding of Nrf2 with ARE in the nucleus
Activation of Nrf2 by ROS-dependent or-independent mechanisms alone is not sufficient to increase the levels of antioxidant enzymes and phase-2-detoxifying enzymes. Activated Nrf2 must bind with ARE in the nucleus in order to increase the expression of many target genes coding cytoprotective enzymes including antioxidant enzymes. This binding ability of activated Nrf2 with ARE in the nucleus was impaired in aged rats [53]. It is unknown whether the binding ability of activated Nrf2 with ARE in the nucleus is impaired in patients with diabetes.
The above studies suggested that activation of Nrf2 would be a useful target for developing agents or drugs for the prevention and improved management of diabetes and diabetic-related complications [71,72]. The levels of antioxidant enzymes and intracellular levels of antioxidant compounds decreased in both animal models of diabetes and in patients with diabetes [73-76]. In order to restore to more normal balance of intracellular defenses, both of these levels should be elevated together. Thus, activation of Nrf2 by a single antioxidant commonly used in the clinical studies may not be sufficient to optimally reduce oxidative stress and chronic inflammation.
Proposed micronutrient mixture for optimally reducing oxidative stress and chronic inflammation
Based on the differential mechanisms of action and cellular distributions of various antioxidants, a micronutrient mixture containing multiple ingredients is proposed. The contents of this mixture include vitamin A, natural mixed carotenoids, vitamin C, vitamin E, curcumin, resveratrol, alpha-lipoic acid, L-carnitine, coenzyme Q10, a synthetic antioxidant N-acetylcysteine (NAC), vitamin D, all B-vitamins, and omega-3-fatty acids. This mixture may reduce oxidative stress by simultaneously enhancing the levels of antioxidant enzymes through activation of the Nrf2 pathway as well as intracellular levels of antioxidant compounds.
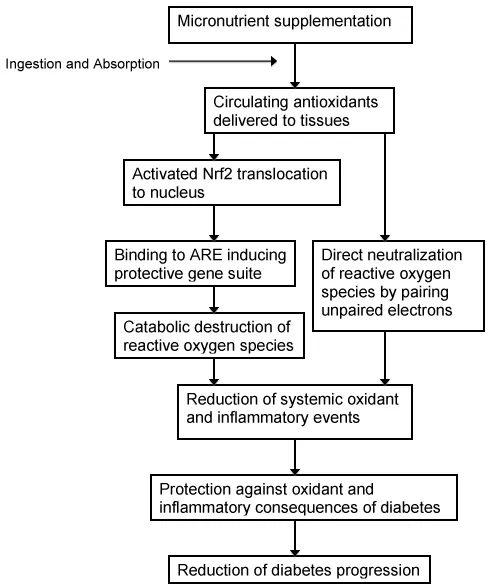
Activation of Nrf2 also suppresses chronic inflammation [77,78]. Some individual antioxidant compounds also reduce chronic inflammation [21-25,79]. Therefore, the proposed micronutrient mixture may also serve to decrease chronic inflammation. The postulated pathways by which diabetes pathology can be restrained, are shown in Figure 1.
Prevention of diabetes
Primary prevention of type 2 diabetes: The purpose of primary prevention is to protect healthy people from developing diabetes type 2. Individuals who are obese with no pre-diabetic conditions are suitable for the primary prevention. The proposed mixture of micronutrients may be effective in reducing the incidence of type 2 diabetes by reducing chronic oxidative stress and inflammation.
Primary Prevention of type 1 diabetes: At present, there are no clear therapeutic approaches to delay the onset of symptoms in children with a family history of diabetes. The mixture of micronutrients described may be effective in delaying the onset of symptoms of diabetes in such children. This possibility is indirectly supported by a laboratory experiment described here.
The gene HOP (TUM-1) is essential for the development of Drosophila melanogaster (fruit fly). Insertion of a mutated HOP (TUM-1) markedly increases the risk of developing a leukemia-like tumor in female flies. In collaboration with Dr. Bhattacharya of NASA, Moffat Field, CA, we observed that whole-body proton irradiation of these flies with proton radiation dramatically increased the incidence of cancer compared to that observed in un-irradiated flies. Treatment with a mixture of antioxidants before and after irradiation blocked the formation of proton radiation-induced cancer in these flies [2]. This finding is of particular interest, because it suggests that heritable genetic basis of the disease can be prevented by antioxidant treatment. The results of this study cannot be extrapolated to the genetic disease in humans.
Secondary prevention of diabetes: The purpose of secondary prevention is to stop or slow the progression of diabetes. Individuals who are pre-diabetic or those who have a family history of diabetes but have not developed symptoms of the disease can be included in secondary prevention. The proposed mixture of micronutrients may be effective in reducing the incidence of diabetes by simultaneously preventing oxidative stress and chronic inflammation.
Improved management of diabetes: Patients with established diabetes are generally on one or more standard medications for improving the treatment of this disease. Combined use of the described proposed described micronutrient mixture in combination with standard therapy may be more effective than confining treatment to maintaining appropriate blood glucose levels.
Diet and lifestyle recommendations: Diet containing plenty of fruits, non-starchy vegetables, complex carbohydrate, and protein, and avoiding sugary drinks are recommended. Lifestyle recommendations include, maintaining normal weight for one’s age and height, refraining from smoking, minimizing caffeine consumption, reducing stress by meditation, yoga, vacation, and daily moderate exercise for 30 minutes. The website of American Diabetes Association is recommended for detailed guidelines concerning diet and lifestyle.
Conclusion
Several studies suggest that increased oxidative stress and chronic inflammation play a key role in the initiation and progression of diabetes. Therefore attenuation of these biochemical defects simultaneously might be needed to prevent and improve the management of diabetes. The body’s defense system against oxidative stress consists of antioxidant enzymes and dietary and endogenous antioxidant compounds. During the extended chronic oxidative stress found patients with diabetes, intracellular antioxidant compounds as well as antioxidant enzymes are present at reduced levels. Single antioxidant compound or antioxidant enzyme mimetic has been commonly used in animal and human studies and these may not achieve optimal benefit in prevention or diabetes treatment. The proposed micronutrient mixture may be more effective means of reducing oxidative stress and chronic inflammation. The multifocal approach of simultaneous enhancement of levels of antioxidant enzymes through activating the Nrf2/ARE pathway, together with increased presence of antioxidant compounds, ensures the broadest protective spectrum for diabetic control.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
The author thanks Professor Stephen C Bondy of University of California at Irvine.
Conflicts
The author is a Chief Scientific Officer of Engage Global, Utah.
- Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24: R453-462. Link: https://goo.gl/lqJrVD
- Prasad KN (2011) Micronutrients for the prevention of diabetes and improvement of the standard therapy. In Micronutrients in Health and Disease Boca raton, Florida: CRC Press 77-102. Link: https://goo.gl/iURz0f
- Stefano GB, Challenger S, Kream RM (2016) Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur J Nutr 55: 2339-2345. Link: https://goo.gl/Skb431
- Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, et al. (2012) Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta 1820: 663-671. Link: https://goo.gl/Z4gMF1
- Giovannini C, Piaggi S, Federico G, Scarpato R (2014) High levels of gamma-H2AX foci and cell membrane oxidation in adolescents with type 1 diabetes. Mutat Res 770: 128-135. Link: https://goo.gl/gdXRCP
- Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF (2015) Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes--Biomarkers as a possible tool for early disease detection for rural screening. Clin Biochem 48: 581-585. Link: https://goo.gl/DKPxId
- Tatsch E, De Carvalho JA, Hausen BS, Bollick YS, Torbitz VD, et al. (2014) Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Mutat Res 782: 17-22. Link: https://goo.gl/PTEbWu
- Saad MI, Abdelkhalek TM, Saleh MM, Kamel MA, Youssef M, et al. (2015) Insights into the molecular mechanisms of diabetes-induced endothelial dysfunction: focus on oxidative stress and endothelial progenitor cells. Endocrine 50: 537-567. Link: https://goo.gl/E9L1zM
- Kowluru RA, Kowluru A, Mishra M, Kumar B (2015) Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res 48: 40-61. Link: https://goo.gl/ea8HPT
- Waris S, Winklhofer-Roob BM, Roob JM, Fuchs S, Sourij H, et al. (2015) Increased DNA dicarbonyl glycation and oxidation markers in patients with type 2 diabetes and link to diabetic nephropathy. J Diabetes Res 2015: 915486. Link: https://goo.gl/rgvuty
- Hussain G, Rizvi SA, Singhal S, Zubair M, Ahmad J (2013) Serum levels of TNF-alpha in peripheral neuropathy patients and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes Metab Syndr 7: 238-242. Link: https://goo.gl/5hKfco
- Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, et al. (2016) Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv 2: e1501332. Link: https://goo.gl/gYA5gJ
- Reinehr T, Karges B, Meissner T, Wiegand S, Stoffel-Wagner B, et al. (2016) Inflammatory Markers in Obese Adolescents with Type 2 Diabetes and Their Relationship to Hepatokines and Adipokines. J Pediatr 173: 131-135. Link: https://goo.gl/hJ5jMG
- Barry JC, Shakibakho S, Durrer C, Simtchouk S, Jawanda KK, et al. (2016) Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep 6: 21244. Link: https://goo.gl/RmVze8
- Morettini M, Storm F, Sacchetti M, Cappozzo A, Mazza C (2015) Effects of walking on low-grade inflammation and their implications for Type 2 Diabetes. Prev Med Rep 2: 538-547. Link: https://goo.gl/ncN8wS
- Karstoft K, Pedersen BK (2016) Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol 94: 146-150. Link: https://goo.gl/N5nkIh
- Singh K, Agrawal NK, Gupta SK, Sinha P, Singh K (2016) Increased expression of TLR9 associated with pro-inflammatory S100A8 and IL-8 in diabetic wounds could lead to unresolved inflammation in type 2 diabetes mellitus (T2DM) cases with impaired wound healing. J Diabetes Complications. 30: 99-108. Link: https://goo.gl/r14QCY
- Perlman AS, Chevalier JM, Wilkinson P, Liu H, Parker T, et al. (2015) Serum Inflammatory and Immune Mediators Are Elevated in Early Stage Diabetic Nephropathy. Ann Clin Lab Sci 45: 256-263. Link: https://goo.gl/3Lo9cC
- Varvarovska J, Racek J, Stozicky F, Soucek J, Trefil L, Pomahacova R (2003) Parameters of oxidative stress in children with Type 1 diabetes mellitus and their relatives. J Diabetes Complications 17: 7-10. Link: https://goo.gl/dlzRqB
- Matteucci E, Rosada J, Pinelli M, Giusti C, Giampietro O (2006) Systolic blood pressure response to exercise in type 1 diabetes families compared with healthy control individuals. J Hypertens 24: 1745-1751. Link: https://goo.gl/PHT49v
- Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, et al. (2007) Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr 86: 1392-1398. Link: https://goo.gl/wdx9WB
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A (2008) Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol 73: 399-409. Link: https://goo.gl/jZN1ie
- Lee HS, Jung KK, Cho JY, Rhee MH, Hong S, et al. (2007) Neuroprotective effect of curcumin is mainly mediated by blockade of microglial cell activation. Pharmazie 62: 937-942. Link: https://goo.gl/lw0TK7
- Rahman S, Bhatia K, Khan AQ, Kaur M, Ahmad F, et al. (2008) Topically applied vitamin E prevents massive cutaneous inflammatory and oxidative stress responses induced by double application of 12-O-tetradecanoylphorbol-13-acetate (TPA) in mice. Chem Biol Interact 172: 195-205. Link: https://goo.gl/euhzXU
- Zhu J, Yong W, Wu X, Yu Y, Lv J, et al. (2008) Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun 369: 471-477. Link: https://goo.gl/BwzyYR
- Vile GF, Winterbourn CC (1988) Inhibition of adriamycin-promoted microsomal lipid peroxidation by beta-carotene, alpha-tocopherol and retinol at high and low oxygen partial pressures. FEBS Lett 238: 353-356. Link: https://goo.gl/4QXUPu
- Niki E (1987) Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci 498: 186-199. Link: https://goo.gl/22Y5bD
- Prasad KN (2016) Oxidative Stress and Pro-Inflammatory Cytokines may Act as one of signals for Regulating MicroRNAs Expression in Alzheimer'sdisease. Mech Ageing Dev In Press. Link: https://goo.gl/Oe8DTy
- Wu H, Kong L, Tan Y, Epstein PN, Zeng J, et al. (2016) C66 ameliorates diabetic nephropathy in mice by both upregulating NRF2 function via increase in miR-200a and inhibiting miR-21. Diabetologia 59: 1558-1568. Link: https://goo.gl/cmplXM
- Yamagishi SI, Matsui T (2016) Protective role of sulphoraphane against vascular complications in diabetes. Pharm Biol 54: 2329-2339. Link: https://goo.gl/tXVg8K
- Alam MM, Iqbal S, Naseem I (2015) Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch Biochem Biophys 584: 10-19. Link: https://goo.gl/PsjXA6
- Parmar MS, Syed I, Gray JP, Ray SD (2015) Curcumin, Hesperidin, and Rutin Selectively Interfere with Apoptosis Signaling and Attenuate Streptozotocin-Induced Oxidative Stress-Mediated Hyperglycemia. Curr Neurovasc Res 12: 363-374. Link: https://goo.gl/WYVu5D
- Bagul PK, Banerjee SK (2015) Application of resveratrol in diabetes: rationale, strategies and challenges. Curr Mol Med 15: 312-330. Link: https://goo.gl/1dFIFc
- Scaramuzza A, Giani E, Redaelli F, Ungheri S, Macedoni M, et al. (2015) Alpha-Lipoic Acid and Antioxidant Diet Help to Improve Endothelial Dysfunction in Adolescents with Type 1 Diabetes: A Pilot Trial. J Diabetes Res 2015: 474561. Link: https://goo.gl/Y2VyKd
- Ellulu MS, Rahmat A, Patimah I, Khaza'ai H, Abed Y (2015) Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther 9: 3405-3412. Link: https://goo.gl/nXhIyY
- Ellulu MS, Khaza'ai H, Patimah I, Rahmat A, Abed Y (2016) Effect of long chain omega-3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Food Nutr Res 60: 29268. Link: https://goo.gl/t441ml
- Montano SJ, Grunler J, Nair D, Tekle M, Fernandes AP, et al. (2015) Glutaredoxin mediated redox effects of coenzyme Q10 treatment in type 1 and type 2 diabetes patients. BBA Clin 4: 14-20. Link: https://goo.gl/vWVbxQ
- Thazhath SS, Wu T, Bound MJ, Checklin HL, Standfield S, et al. (2016) Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 103: 66-70. Link: https://goo.gl/128ZkQ
- Mao J, Bath SC, Vanderlelie JJ, Perkins AV, Redman CW, et al. (2016) No effect of modest selenium supplementation on insulin resistance in UK pregnant women, as assessed by plasma adiponectin concentration. Br J Nutr 115: 32-38. Link: https://goo.gl/Be3S2t
- Yin RV, Phung OJ (2015) Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutr J 14: 14. Link: https://goo.gl/JW9Nse
- Prasad KN (2016) Simultaneous activation of Nrf2 and elevation of antioxidant compounds for reducing oxidative stress and chronic inflammation in human Alzheimer's disease. Mech Ageing Dev 153: 41-47. Link: https://goo.gl/PO0Exi
- Jaramillo MC, Zhang DD (2013) The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 27: 2179-2191. Link: https://goo.gl/4dGTRO
- Williamson TP, Johnson DA, Johnson JA (2012) Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology 33: 272-279. Link: https://goo.gl/yExXR6
- Lee D, Kook SH, Ji H, Lee SA, Choi KC, et al. (2015) N-acetyl cysteine inhibits H2O2-mediated reduction in the mineralization of MC3T3-E1 cells by down-regulating Nrf2/HO-1 pathway. BMB Rep 48: 636-641. Link: https://goo.gl/BFcRFU
- Romanque P, Cornejo P, Valdes S, Videla LA (2011) Thyroid hormone administration induces rat liver Nrf2 activation: suppression by N-acetylcysteine pretreatment. Thyroid 21: 655-662. Link: https://goo.gl/09wPeK
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, et al. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313-322. Link: https://goo.gl/kcEc3j
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, et al. (2000) The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochemical Society transactions 28: 33-41. Link: https://goo.gl/VbpTBm
- Chan K, Han XD, Kan YW (2001) An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA 98: 4611-4616. Link: https://goo.gl/I7CJqH
- Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, et al. (2007) Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66: 75-85. Link: https://goo.gl/FEs3f0
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, et al. (2009) Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A 106: 2933-2938. Link: https://goo.gl/wKSsZh
- Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rabano A, et al. (2012) alpha-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Hum Mol Genet 21: 3173-3192. Link: https://goo.gl/pgU3wP
- Xi YD, Yu HL, Ding J, Ma WW, Yuan LH, et al. (2012) Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from beta-amyloid peptide-induced oxidative damage. Curr Neurovasc Res 9: 32-41. Link: https://goo.gl/LxnUlR
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, et al. (2004) Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A 101: 3381-3386. Link: https://goo.gl/0x3WJH
- Trujillo J, Chirino YI, Molina-Jijon E, Anderica-Romero AC, Tapia E, et al. (2013) Renoprotective effect of the antioxidant curcumin: Recent findings. Redox biology 1: 448-456. Link: https://goo.gl/WbORq1
- Steele ML, Fuller S, Patel M, Kersaitis C, Ooi L, Munch G (2013) Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol 1: 441-445. Link: https://goo.gl/S3rTSH
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, et al. (2008) Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L478-488. Link: https://goo.gl/Qe6E3x
- Gao L, Wang J, Sekhar KR, Yin H, Yared NF, Schneider SN, et al. (2007) Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J Biol Chem 282: 2529-2537. Link: https://goo.gl/gJWyI0
- Saw CL, Yang AY, Guo Y, Kong AN (2013) Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol 62: 869-875. Link: https://goo.gl/esRf1m
- Song J, Kang SM, Lee WT, Park KA, Lee KM, et al. (2014) Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression. Exp Neurobiol 23: 93-103. Link: https://goo.gl/UvqFQ0
- Ji L, Liu R, Zhang XD, Chen HL, Bai H, et al. (2010) N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol 22: 535-542. Link: https://goo.gl/6ciqpm
- Choi HK, Pokharel YR, Lim SC, Han HK, Ryu CS, et al. (2009) Inhibition of liver fibrosis by solubilized coenzyme Q10: Role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol Appl Pharmacol 240: 377-384. Link: https://goo.gl/JYE3Ns
- Im N-K, Zhou W, Na M, Jeong G-S (2014) Pierisformoside B exhibits neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells via the HO-1/Nrf2-mediated pathway. Int Immunopharmacol 24: 353-360. Link: https://goo.gl/hVo6Wg
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, et al. (2006) Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127: 59-69. Link: https://goo.gl/5HzsPw
- Li XH, Li CY, Lu JM, Tian RB, Wei J (2012) Allicin ameliorates cognitive deficits ageing-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Neurosci Lett 514: 46-50. Link: https://goo.gl/D7lQo1
- Bergstrom P, Andersson HC, Gao Y, Karlsson JO, Nodin C, Anderson MF, et al. (2011) Repeated transient sulforaphane stimulation in astrocytes leads to prolonged Nrf2-mediated gene expression and protection from superoxide-induced damage. Neuropharmacology 60: 343-353. Link: https://goo.gl/KaI5Ky
- Wruck CJ, Gotz ME, Herdegen T, Varoga D, Brandenburg LO, et al. (2008) Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol Pharmacol 73: 1785-1795. Link: https://goo.gl/c7dPgi
- Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843-854. Link: https://goo.gl/nDAuQi
- Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855-862. Link: https://goo.gl/p9wvqJ
- Macfarlane LA, Murphy PR (2010) MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics 11: 537-561. Link: https://goo.gl/eygC3X
- Londin E, Loher P, Telonis AG, Quann K, Clark P, et al. (2015) Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc Natl Acad Sci U S A 112: E1106-1115. Link: https://goo.gl/HzF9d4
- Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM (2015) The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacol Res 91: 104-114. Link: https://goo.gl/fW862i
- Li B, Liu S, Miao L, Cai L (2012) Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res 2012: 216512. Link: https://goo.gl/OEjzOn
- Gawlik K, Naskalski JW, Fedak D, Pawlica-Gosiewska D, Grudzien U, et al. (2016) Markers of Antioxidant Defense in Patients with Type 2 Diabetes. Oxid Med Cell Longev 2016: 2352361. Link: https://goo.gl/p1MAlV
- Takitani K, Inoue K, Koh M, Miyazaki H, Kishi K, et al. (2014) alpha-Tocopherol status and altered expression of alpha-tocopherol-related proteins in streptozotocin-induced type 1 diabetes in rat models. J Nutr Sci Vitaminol (Tokyo) 60: 380-386. Link: https://goo.gl/JrL6OA
- Araki A, Yoshimura Y, Sakurai T, Umegaki H, Kamada C, et al. (2016) Low intakes of carotene, vitamin B2 , pantothenate and calcium predict cognitive decline among elderly patients with diabetes mellitus: The Japanese Elderly Diabetes Intervention Trial. Geriatr Gerontol Int. Link: https://goo.gl/qo9ZmZ
- Bukhari SA, Naqvi SA, Nagra SA, Anjum F, Javed S, et al. (2015) Assessing of oxidative stress related parameters in diabetes mellitus type 2: cause excessive damaging to DNA and enhanced homocysteine in diabetic patients. Pak J Pharm Sci 28: 483-491. Link: https://goo.gl/4ccxNl
- Li W, Khor TO, Xu C, Shen G, Jeong WS, et al. (2008) Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76: 1485-1489. Link: https://goo.gl/xxjs0Z
- Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690: 12-23. Link: https://goo.gl/P0RjZH Abate A, Yang G, Dennery PA, Oberle S, Schroder H (2000) Synergistic inhibition of cyclooxygenase-2 expression by vitamin E and aspirin. Free Radic Biol Med 29: 1135-1142. Link: https://goo.gl/f4KIJV
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
