Annals of Molecular and Genetic Medicine
UV-visible spectroscopic investigations on the post-irradiation storage effect of polystyrene
P Vivek Vardhan1, Chitra Shaji2, Alok Sharan3 and Lata I Shukla4*
2Department of Physics, Pondicherry University, Pondicherry – 605014, India
3Professor, Department of Physics, Pondicherry University, Pondicherry – 605014, India
4Professor, Department of Biotechnology, School of Life Sciences, Pondicherry University, Pondicherry – 605014, India
Cite this as
Vardhan PV, Shaji C, Sharan A, Shukla LI (2024) UV-visible spectroscopic investigations on the post-irradiation storage effect of polystyrene. Ann Mol Genet Med 8(1): 001-008. DOI: 10.17352/amgm.000013Copyright License
© 2022 Du K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Polystyrene is an electron-rich polymer wherein the electrons ejected on irradiation are localized and could be investigated by using spectroscopic methods. Pre-irradiated, PTPC (Pre-irradiated Tarsons Polystyrene Control) were irradiated with a cumulative dose of γ-rays 51, 77 and 129 kGy at 300-308 K referred to as RTPS (Re-irradiated Tarsons Polystyrene Samples). These RTPS were subjected to different post-irradiation treatments and were analyzed by UV-Vis absorption spectrometry. The regions 300 – 400 and 600 – 900 nm show differential absorption in RTPS in dose dose-dependent manner. The energy is absorbed by the phenyl chromophores of PS and redistributes the excitation energy. The data clearly suggests that RTPS (1mm) has retained its structural and chemical integrity as evidenced by UV-Vis spectrometry and hydrogen ion concentrations. The sterility was maintained for prolonged periods until packed properly facilitating the re-use multiple times before discarding. Though γ-irradiation has been in use for sterilization for a long time. The applications include the reusability of PS materials and evidence is provided for the structural and chemical integrity after re-irradiation. This method is cost-effective for biological laboratories and environmentally friendly.
Introduction
Polystyrene (PS) is a petroleum-based plastic that is the most preferred material for laboratory plastic ware used in biological, medical, and agricultural research. It is also one of the most commonly used plastics in the world, with a turnover of around a billion kilograms produced each year. Over the past six decades, the production and use of plastics worldwide have increased at an average annual rate of about 8%, attaining an all-time maximum in 2014, of 300 million tonnes. By simple extrapolation of historical data, it can be predicted that by 2020 production will exceed 420 million tonnes [1]. Despite the fact that researchers represent a tiny fraction of the world’s population, life science researchers are far from innocent when it comes to contributing to the generation of global plastic waste. Some 20,500 institutions worldwide are involved in life science research where plastic disposal is likely to be the heaviest [2]. Of the 300 million tonnes of global plastic waste generated in 2014, these labs could have generated up to 5.5 million tonnes which could equate to around 2% of the total mound. This exponential growth has led to increasing amounts of plastic waste which is being discarded, ultimately ending up in landfills or being incinerated releasing harmful chemicals into the atmosphere. This raises serious concern among scientists and organizations worldwide for sustainability compelling the reduction of plastic waste by encouraging the reuse and recycling of end-of-life products.
Reducing various types of plastic waste being generated and its management is currently the most popular area of interest in sustainable development and waste management. The comparative Life Cycle Assessment (LCA) studies of various single-use vs. reusable plastic products such as cups, plates, bowls, and bottles reported that reusing has overall least global warming potential and total energy consumption than single-use products [3]. Sustainable development implies reducing the rate of extraction of natural resources. Hence, by reusing the PS lab ware by cold sterilization using γ-radiation, we can save resources and limit the amount of hazardous chemical emissions into the atmosphere.
PS is an electron-rich polymer that can withstand higher doses of ionizing radiation due to its aromatic ring structure. The unique feature associated with PS is that the photo-oxidation of PS does not show autocatalytic behavior typical for auto-oxidation of polymers [4]. The secondary and tertiary carbon atoms of the PS backbone are potential sites of oxidation and chain scission [5]. Thus, the structure of PS enables the energy transfer from excited phenyl groups to photo-reactive products. This makes it rather easy for the free radicals to travel long distances away from the site of generation leading to radical-radical recombination. Photo oxidation of PS irradiated with UV at 253.7 nm showed the formation of polyenes, hydrogen peroxide, and ketones along with CO2 without any permanent chain scission [6]. Only 4% of absorbed oxygen results in chain scissions. The absorption of UV light by the phenyl chromophores of PS leads to the energy transfer to photo-reactive products.
It may be noted that for the polymers, poly-oxy-methylene [7] and PS-Fe composites [8] an increase in brittleness with higher doses (50 kGy) irradiation was observed. In the case of segmented polyurethane, the γ-irradiated samples under different conditions have been studied by FT-IR, Gel Permeation Chromatography, X-ray Diffraction, and Small Angle Neutron Scattering [9]. An increased hard domain distance by 70% - 80% for samples irradiated with 500 kGy and also the formation of larger linked structures was seen. The polypropylene irradiated with 20 – 100 kGy showed a reduction in free radicals in addition to mobilizing additives, as detected by Electron Spin Resonance [10]. This reduces the extent of polymer oxidation and greatly improves the mechanical properties of the polymer.
IR spectroscopic study of PS suggests an increase in C = O and O – H groups on exposure to γ-radiation in the presence of oxygen [11]. The various radiolysis products formed on irradiation with 25 kGy have been reported by Buchalla, et al. [12] which provides an insight into the molecular mechanisms of degradation of PS. By absorbing the UV-Vis light electrons are promoted from their ground state to an excited state. The absorption of 305nmbenzyl radical has been interpreted in terms of vibrational frequencies similar to the ultraviolet absorption of toluene. A new spectrum of the phenoxyl radical in the region of 600nm is described and assigned to a π ← n transition [13].
In this study, one-time used PTPC (Pre-irradiated Tarsons Polystyrene Control) were further subjected to cumulative doses of irradiation (51, 77, and 129kGy) referred to as RTPS (Re-irradiated Tarsons Polystyrene Samples). The RTPS was subjected to post-irradiation treatments with a view to identifying the changes in the UV-Vis absorption under different storage conditions (Figure 1). The UV-Vis spectrometry gives the structural and chemical modifications in the material as a result of γ-irradiation. It is essential to assess the release of H+ ions into the solution inside the irradiated material as pH plays an important role in biological systems. Also, we assessed the sterility by monitoring the microbial growth under aseptic conditions. Identifying the effective radiation dose suitable for sterilization of pre-molded PS without adversely affecting its properties to reuse is the main goal of this study.
Materials and methods
Test material
The sample preparation, irradiation, and post-irradiation treatments were performed as given by Vardhan and Shukla [11]. Briefly, the sterile disposable PS petri plates (thickness: 1 mm; diameter: 90 mm, sterilized with 25 kGy) were purchased from Tarsons Products Pvt. Ltd., India for plant tissue culture experiments in our laboratory. The one-time-used plates were used for this study referred to as PTPC (Pre-irradiated Tarsons Polystyrene Control). These PTPC samples were further subjected to γ-irradiation with cumulative doses 51, 77 and 129 kGy, referred to as RTPS (Re-irradiated Tarsons Polystyrene Samples). These were irradiated in 60Co Gamma chamber 5000(BARC, Mumbai, India) with a dose rate of 3.5 kGyh-1. These were either stored in the dark for 24h, 48h, and 2160h (90 days) or exposed to the sun outdoors (120,000 lx, 37 °C) for 30 days.
UV-Vis spectrometry of RTPS
The control (PTPC) and samples (RTPS) were cut into 4 cm x 2 cm clear scratch-free rectangular pieces and absorption spectra were recorded using a UV-Vis spectrophotometer (Ocean Optics Germany, HR4000CG-UV-NIR). The spectra of RTPS were recorded either immediately (0h) or after post-irradiation storage under different conditions. The absorbance was recorded for wavelength range 200 nm to 1100 nm. The data were obtained by the software Ocean Optics Spectra Suite (Germany). For each recording, the background signal and the reference (PTPC) were measured and then subtracted from the spectra of the RTPS sample to plot the results using OriginPro 8.5. All the spectra were recorded in three repeats. The results were analyzed by measuring the relative amplitude percentage (Supplementary Data).
Measurement of hydrogen ion concentration
To evaluate changes in hydrogen ion concentration associated with irradiation, sterile distilled water (20 ml) was poured into the plates (PTPC and RTPS) in Horizontal Laminar Air Flow chamber (Marti Instruments & Chemicals Pvt. Ltd., India) and kept at 300 K for 24 hours. The pH was measured using a calibrated pH meter (Eutech Instruments). The initial pH was recorded and monitored for subsequent changes after incubation in RTPS. Measurements were done for 5 replicates for all the treatments.
Evaluating sterility of RTPS
The sterility of the RTPS was evaluated by incubating sterile MS media containing sucrose (3%) and agar (0.8%) with a pH of 5.7. The media was sterilized (autoclaved at 393K, with pressure 121 psi for 20min), air-cooled to 333K, and poured into RTPS in sterile conditions within the Horizontal Laminar Air Flow chamber (Matri Instruments & Chemicals Pvt. Ltd., Pondicherry). After the media was solidified, the plates were sealed and incubated in the dark for 45 days in a plant incubator maintained aseptically at 298K (SANYO Cooled Incubator MIR-554, Japan).
Results
UV-Vis absorption
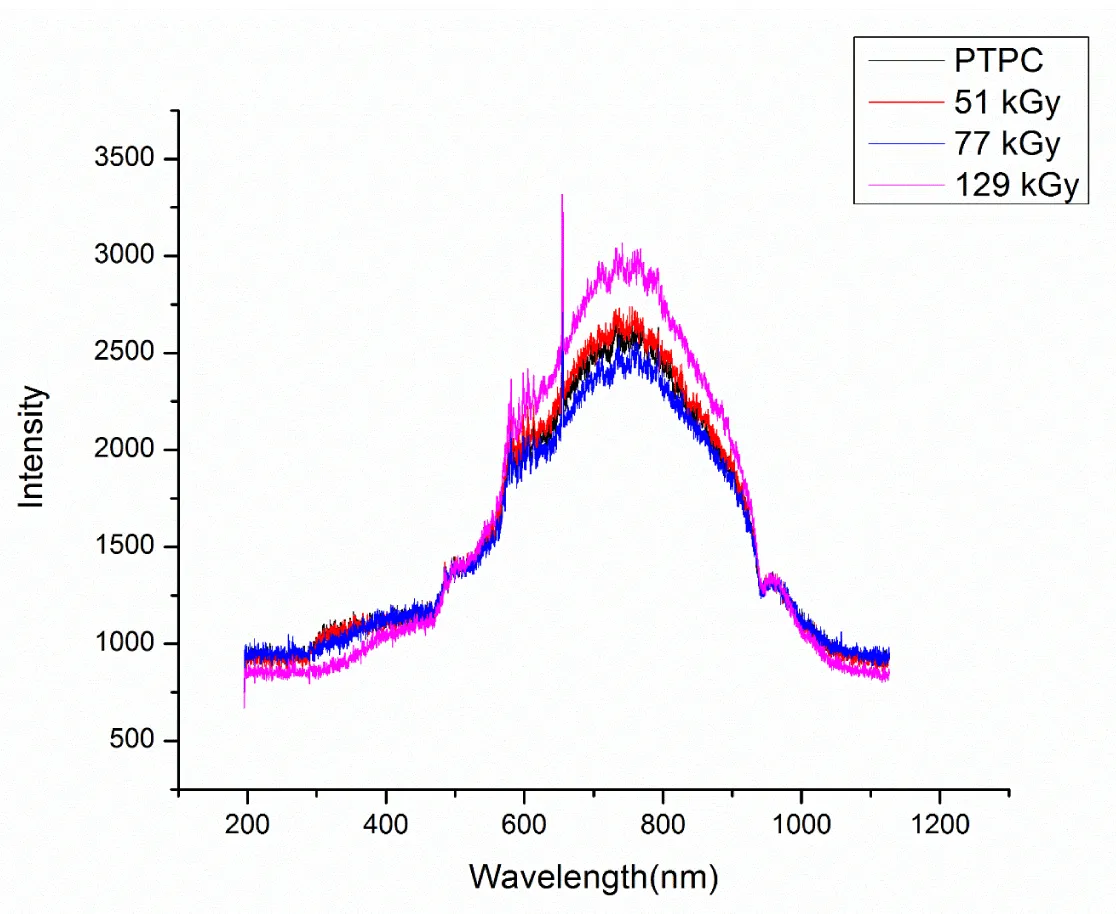
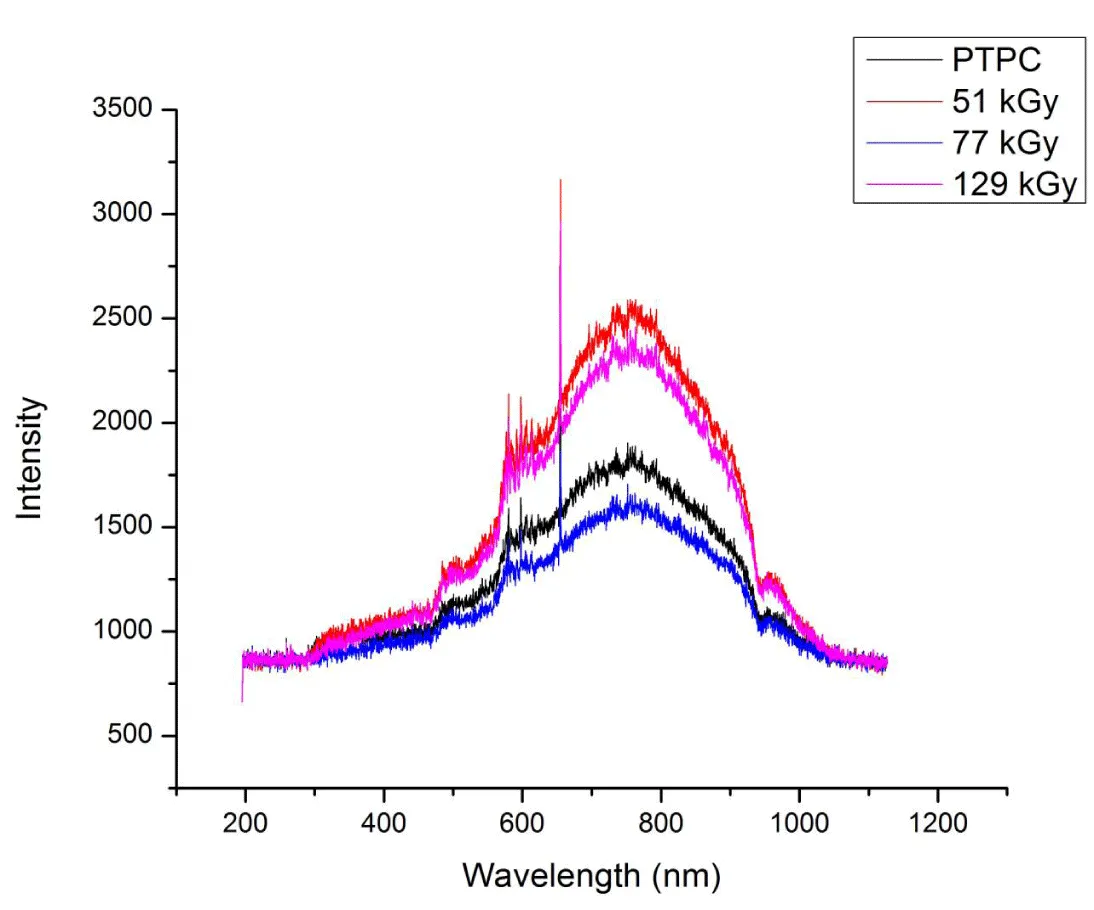
Effect of gamma irradiation on UV-Vis spectra of RTPS: The UV-Vis spectra of RTPS were recorded from 200 – 1100 nm (Figure 2). The absorption spectra could be divided into 300 – 400, 400 – 600, 600 – 900 and 900 – 1000 nm. The region from 200 – 300 nm showed a flat plateau for all the RTPS irrespective of the irradiation dose. The absorption is higher in the region 600 – 900 nm with absorption maxima at 750 nm.
The UV-Vis spectra of RTPS were recorded immediately (<10 min) after irradiation (51, 77 and 129 kGy). The regions from 400 – 600 and 900 - 1000 nm are seen unaffected irrespective of the dose (Table 1). However, a gradual decrease in absorption is seen with the increase in dose at 300 – 400 nm, where 129 kGy showed maximum reduction (28%) and 77 kGy showed significant reduction (10%). At 600 – 900 nm, the reduction is not uniform where 77 kGy showed maximum reduction (7%) and 129 kGy showed maximum enhancement (17%). Hence the spectra showed dose dose-dependent decrease in absorption for RTPS 77 and 129 kGy at 300 – 400 and 600 – 900 regions.
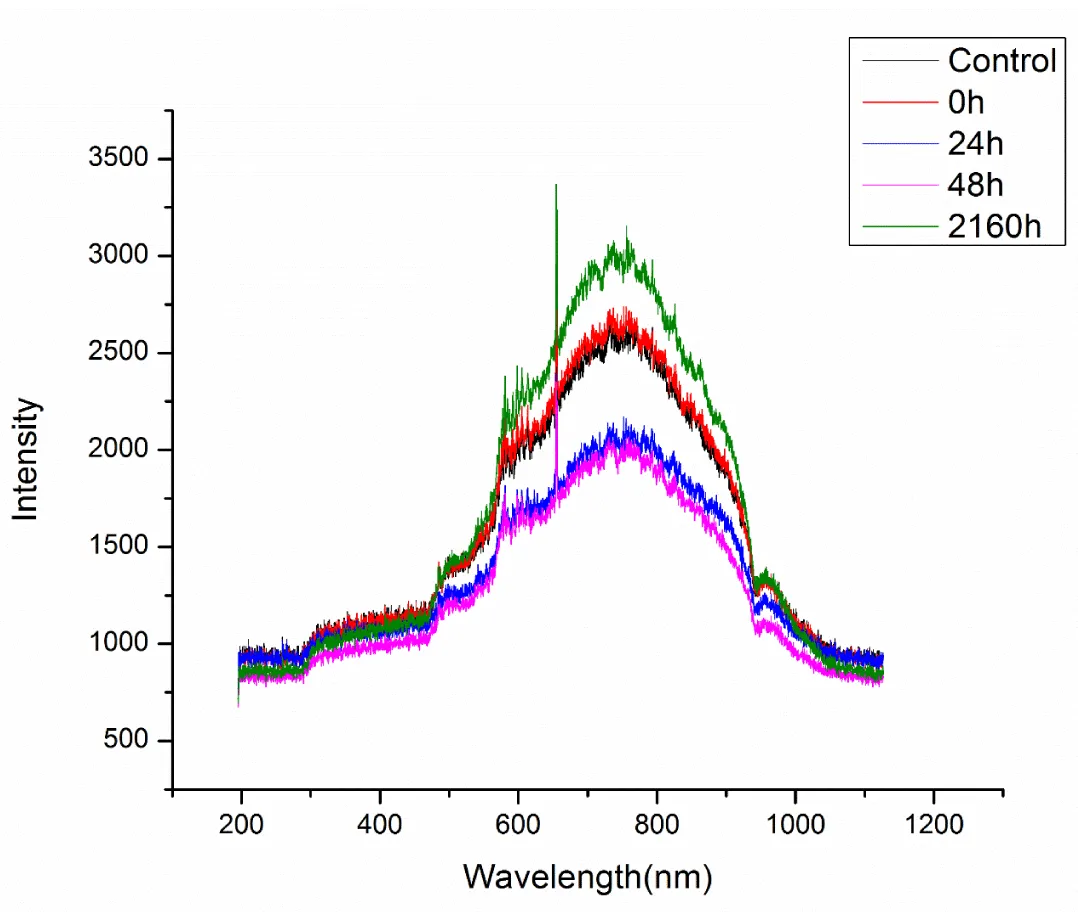
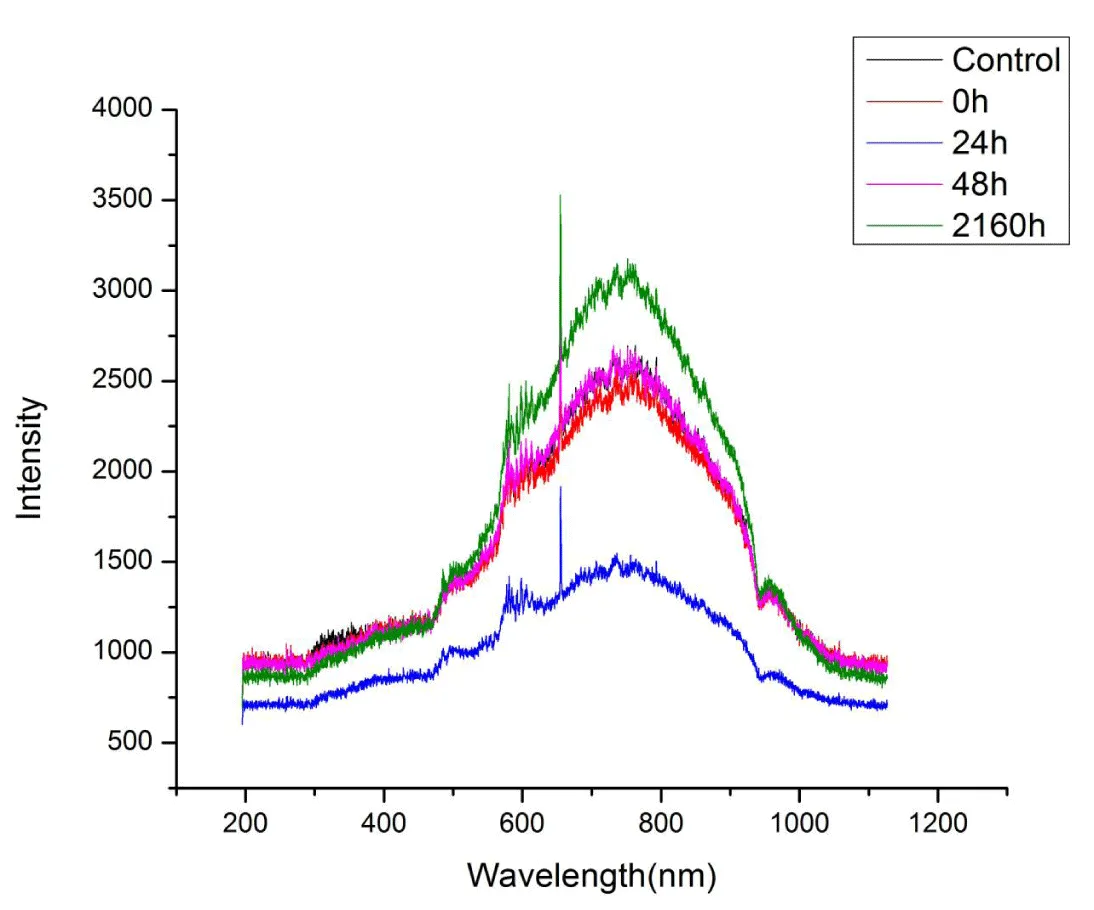
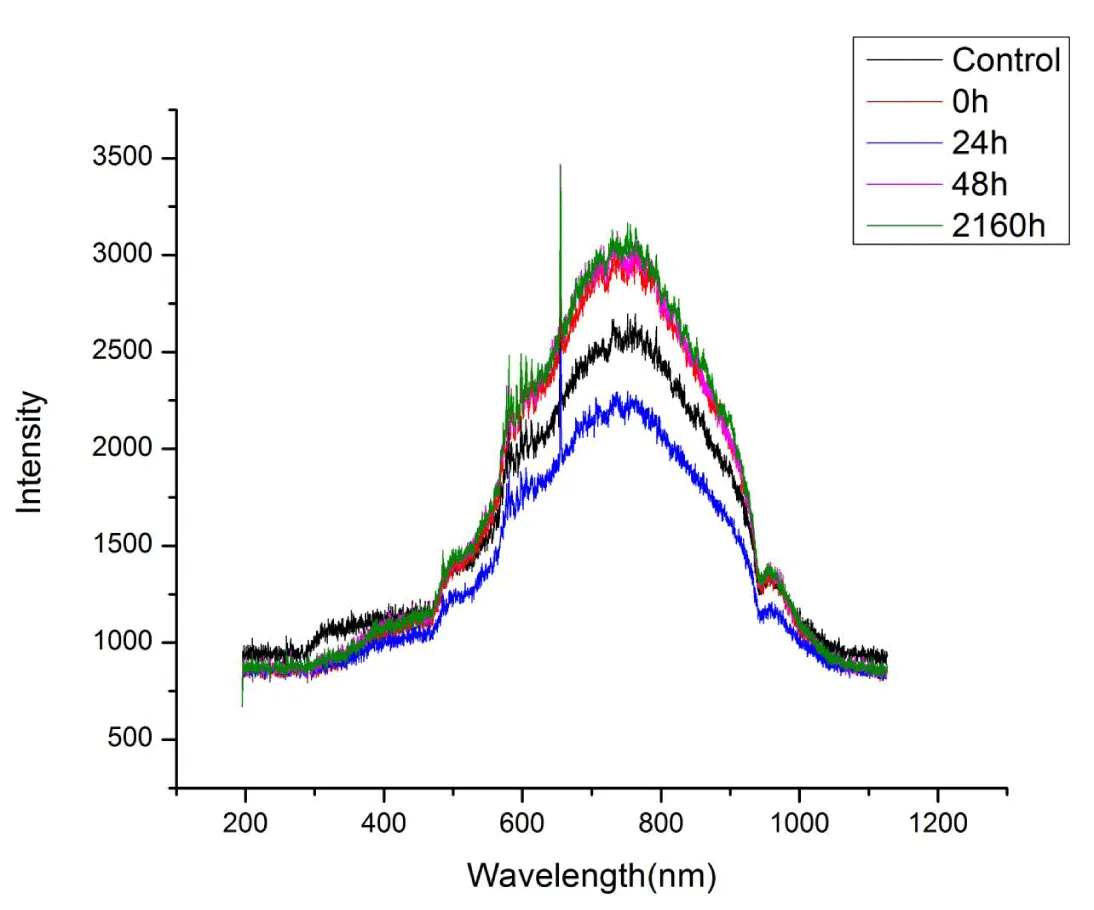
Effect of post-irradiation storage on UV-Vis spectra of RTPS: The effect of post-irradiation storage (0h, 24h, 48h, 2160h) on UV-Vis spectra of RTPS (51, 77 and 129 kGy) in dark at 300-308Kis given in Figures 3-5. As a result of storage, a general pattern of reduction of absorbance and a reversion to normal on prolonged storage (2160h) was observed for all the doses at 300 – 400 and 600 – 900 nm (Tables 2-4). Specifically, for 51 kGy, at 300 – 400 nm, the 24h storage showed a significant reduction (14%) and 48h storage showed maximum reduction (23%). At 600 – 900nm, both 24 and 48h storage showed maximum reduction (~20%). For 77 kGy, the 24-hour storage showed a significant reduction (30%) at 300 – 400 nm and a maximum reduction (46%) at 600 – 900 nm. Also, for 129 kGy, the 24h storage showed significant reduction at both 300 – 400 and 600 – 900 nm (20% and 14% respectively). On prolonged storage (2160h), all the RTPS (51, 77, and 129 kGy) showed UV-Vis spectra comparable with that of the control.
Effect of heat treatment on UV-Vis spectra of RTPS: Some of the RTPS samples were exposed to heat (1,20,000 lx, 37 °C) after irradiation and UV-Vis spectra were recorded (Figure 6). The overall spectral pattern for all heat-treated RTPS samples (51, 77, and 129 kGy) showed an increase in intensity with respect to the PTPC. Specifically, at 600 – 900 nm, 51 kGy showed maximum increase (45%). At 900 – 1000 nm, both 51 and 129 kGy showed a significant increase (22%) in absorption (Table 5).
Hydrogen ion concentration
The distilled water was poured into the RTPS (51, 77, and 129 kGy) and kept overnight. Its initial pH was 6.1 and after incubation in the RTPS, a slight reduction in the pH is noted ~5.5 (Table 6). The RTPS showed a pH ranging from 6.2 to 5.4. Only 24h stored RTPS showed a slight reduction in pH (~5.5). All the 48 and 2160 h stored RTPS (51, 77, and 129 kGy) showed pH similar to that of the initial value (~6.0). Similar results were seen for heat-treated RTPS.
Evaluation of sterility of RTPS
In the PTPC, a microbial infestation of bacteria and fungi was seen on the first-day post-inoculation on MS media (Table 7). However, in RTPS irradiated with 51, 77, and 129 kGy no microbial colonies were seen even after 45 days after incubation.
Financial analysis
The amount of waste generated by the life science laboratories on the basis of Petri dishes alone was estimated. For our department with nine principal investigators, each with an average of six students, we use more than 10 packs of Petri dishes per laboratory per year. In our university with ten life science departments, we spend Rs. 2.4 million ($34,604) and generate 6.75 tonnes of waste annually on Petri dishes alone. There are 903 UGC-recognized universities in India, which could correspond to Rs. 2145.5 million ($31 million) and 6095 tonnes of waste per year. These values did not include other institutions funded under various schemes of government and private organizations. Some 20,500 institutions worldwide are involved in biological, medical, or agricultural research. So life science laboratories around the world are spending Rs. 48.7 billion ($700 million) and generating 1,38,375 tonnes of waste annually on Petri dishes alone. Therefore, the reuse of Petri dishes by γ-irradiation would reduce their consumption by 3 folds and save Rs. 32.6 billion ($500 million) and 92,711 tonnes of waste generation per year globally.
Discussion
UV-Vis absorption
Effect of gamma irradiation on UV-Vis spectra of RTPS: The RTPS is distinct in that the polystyrene (PS) is oxidized and its reusability by γ-irradiation is dependent on the physical, chemical, and biological parameters. The FT-IR investigations on RTPS, which provided interesting data on modifications in bonding patterns have been reported (Vardhan and Shukla 2018) [9]. Briefly, it suggests the enhancement of C = O and O – H bonds on γ-irradiation under different storage conditions. However, information on the free radical reactions and structural modifications of PS due to γ-irradiation could be provided by UV-Vis absorption studies. The RTPS absorption range is from 300 – 900 nm with maximum absorption at 750 nm (Figure 2). The UV-Vis spectra of PTPC and RTPS could be due to various radiolysis products formed which are trapped post-irradiation in the PS polymer and these could react with oxygen [12]. McGovern, et al. [14] reported absorption at 280 – 291 nm regardless of the molecular weight of the virgin PS. Also, Newell [15] measured five virgin PS and observed that neither molecular weight nor variables of polymerization reaction affected the UV-Vis absorption at shorter wavelengths.
In the UV region, the RTPS showed an absorption pattern different from Geuskens, et al. [6], where absorption maxima at 240 nm are reported. The investigations of Li, et al. [16] on virgin PS are quite evident that the absorption at longer wavelengths of UV region is due to the associative interactions among the phenyl groups of PS. However, no absorption was observed in this region possibly due to its rigidity and total oxidation of RTPS into simpler fragments. The UV-Vis spectra of RTPS showed dynamic changes in absorption at 300 – 400 and 600 – 900 nm as a result of irradiation (Table 1). It is interesting to note that these changes showed a pattern of a reduction in relative amplitudes at 350 nm and an increase at 750 nm with the increase in irradiation dose. The increase in absorption in 600 – 900 nm could be related to an increase in the fragmentation of PS containing 6 to 9 monomeric units [6]. They correspond to polyenes and the selective generation of these fragments is noted.
The quantitative study of the chemical reactions resulting from irradiation of PS with UV at 253.7 nm in the presence of oxygen was reported [6]. Their findings include the formation of an acetophenone-type end-group and an unsaturated chain end simultaneously. Unsaturated chain-ends then act as starting points for the building of sequences of conjugated double bonds (polyenes). They also suggested that the changes of absorption in the UV region are associated with the excitation of several types of polyenes simultaneously because their absorption spectra overlap. Also, there is a possible formation of diene (2n), triene (3n), and tetraene (4n) with absorption maxima at 355, 390, 455, and 520 nm and the larger polyenes are reported to be selectively excited.
In our data, dose-dependent change in the amplitudes is observed in the regions of 300 – 400 and 600 – 900 nm which corresponds to dienes and polyenes, respectively. The excited unsaturated groups could decompose with the evolution of hydrogen by a molecular mechanism to yield a conjugated diene. The increase in polyene yield is due to the high efficiency of energy transfer to terminal double bonds which are produced with higher yield in the presence of oxygen. Since energy is absorbed almost exclusively by the phenyl chromophores of the polymer, most of the observed reactions occur only because of energy transfer to photo-reactive products. The influence of dose rate (8.4 kGy/h) and electron beam accelerator (3600 kGy/h) was studied by Rabaeh and Basfar [17] who found the dose-response of dyed PS films with dithizone dye was changed by about 2%.
The presence of aromatic rings in the chemical structure significantly increases the radiation stability of polymers [18]. The mechanism suggested is the redistribution of the excitation energy throughout the material. The RTPS consists of long hydrocarbon chains with an aromatic ring in the repeating unit. On electron beam irradiation, UV spectra showed λmax at 200 nm and an increase in λmax at 250 nm with dose [19]. Consequently, the PS polymer is suggested to be transformed into an organic network structure that could be composed of fused and non-fused Polycyclic Aromatic Hydrocarbons (PAHs) with different cluster sizes. In our data, we found changes in absorption at 300 – 400 and 600 – 900 nm wherein the peaks at 350 and 500 nm correspond to PAHs. Thus the absorption in the UV-Vis spectral region is associated with the different oxidized and chain scission products formed as a result of γ-irradiation. The stabilized and labile products entrapped show different levels of accumulation and dose dependence. The selective accumulation in this region suggests that the stable products of radiolysis of oxidized PS are absorbed in this region.
Effect of post-irradiation storage on UV-Vis spectra of RTPS: The RTPS irradiated with 51, 77, and 129 kGy and stored for different time periods were used to study the effect of post-irradiation storage. The RTPS showed interesting results on post-irradiation storage for 24, 48, and 1260h (90 days). At 300 – 400 nm, all the RTPS (51, 77, and 129 kGy) showed dynamic changes in storage for 24 and 48 h (Supplement Figures 1-4). Although the reduction is dose-dependent, a significant reduction in amplitudes for stored RTPS is observed. At 400 – 600 nm, no dynamic changes were observed for all stored RTPS except on 24h storage a small reduction was seen. At 600 – 900 nm, dynamic changes in amplitudes were observed. For all RTPS (51, 77 and 129 kGy) on storage of 24 and 48 h significant reduction in amplitudes is noted. On prolonged storage (2160h), the amplitudes were increased which are comparable to the PTPC. At 900 – 1000 nm, no dynamic changes were observed. However, a sudden drop of almost 50% is observed only for 24h stored RTPS 77 kGy. At 300 – 400 and 600 – 900 nm, the reduction in relative amplitudes on storage of 24 and 48 h is noted for the RTPS in this order
Control > 51 kGy ≈ 77 kGy > 129 kGy
This could be associated with the interaction of the radiation-induced precursors with the electron-rich PS backbone and the transfer of charge across the backbone. This appears not to be the case for RTPS 129 kGy, where post-irradiated storage shows slight accumulation and the moieties are trapped.
Interestingly, on prolonged storage, in all the RTPS (51, 77, and 129 kGy), a reverting trend is observed where the relative amplitudes were increased making it comparable with that of PTPC (Tables 2 – 4). The RTPS 51 kGy showed an increase in amplitude by 23% at 600 – 900 nm. The same pattern is also seen for 77 kGy and 129 kGy where an increase of 21% and 20% is noted, respectively. Thereby, suggesting that the moieties absorbing at 300 – 400 and 600 – 900 nm are accumulated provided by the prolonged storage.
This reduction in the amplitudes of various wavelengths other than 600 – 900 nm suggests that it is essential to probe the mechanisms underlying the development of the radiation-induced changes in oxidized PS. The absorption of PS after photo-oxidation resulted in the formation of polyenes, hydrogen peroxides, ketones, and CO2 but no permanent scission [6]. The authors proposed the formation of photo-reactive products on irradiation with UV at 253.7 nm. The phenyl chromophores of the PS thereby absorb the energy and the charge transfer interactions occur with the radiation-induced precursors. The γ-irradiation on the other hand is an ionizing radiation that causes direct and indirect effects.
Effect of heat treatment on UV-Vis spectra of RTPS: The overall spectral pattern of heat-treated RTPS showed an increase in intensity specifically at 600 – 900 and 900 – 1000 nm (Figure 6). However, only the heat-treated RTPS77 kGy showed a reduction in amplitudes (Table 5). Since the RTPS used in this study is already oxidized and further irradiated, it is quite likely that the tightly bound PS polymer is undergoing changes resulting in the UV spectral features reported herein. The effect of oxygen saturation and thermal treatment post-irradiation could play an important role in the susceptibility to radiation damage of plastics. The pre-irradiation conditions such as the presence of oxygen and the temperature in which it is stored create a complex set of effects on the susceptibility to radiation damage [20]. Hence, it is possibly due to the accumulated moieties that are trapped being made available on heat treatment. Also, it is found that on treatment with intense sunlight, degradation occurs preferably by the molecular chains scission and no crosslinking was produced [21]. However, they found a reduction in molecular mass as a function of exposure time on the surface.
Hydrogen ion concentration
We have studied the effect of irradiation dose and storage on the release of H+ ions into the solution from the RTPS (Table 6). The release of H+ ions from the polymer, thereby a slight reduction in pH of water is observed for 24h stored RTPS. However, the 48-hour stored RTPS does not show any change in pH regardless of the irradiation dose. It indicates that the loss of hydrogen atoms from PS chains could lead to the formation of a stable radiolysis product. The same pattern was observed for prolonged storage and heat-treated RTPS. Hence, the data clearly suggests that the RTPS does not liberate H+ ions after 48 h of post-irradiation storage.
In the radiolysis of solid polymers, molecular hydrogen is the main constituent of the low molecular weight debris of the degraded polymer. The hydrogen gas released from the γ-irradiated PS is reported by Zagorski and Gluszewski [22]. Much of the subsequent chemistry is due to the carbon-centered radical and the hydrogen atom produced in this process. Also, the formation of molecular hydrogen is obviously connected with the breakage of the C – H bonds which are readily broken in radiolysis. The yield of hydrogen can be used to indicate the C – H bond breakage by radiolysis [23]. The enhanced conductivity and the dielectric constant for irradiated polystyrene composites were reported by Abu Saleh, et al [24].
Evaluation of sterility of RTPS
For un-irradiated PTPC, the fungal contamination was observed after 1 day of post-inoculation followed by bacterial colonies (Table 7). On the other hand, for RTPS, no microbial contamination is observed even after 45 days. This is due to sterilization wherein, the microbes and spores present are killed during the process of irradiation thereby even after 45 days no growth was observed. γ-irradiation is a physical means of decontamination because it kills microbes by breaking down DNA, inhibiting microbial growth. Due to its short wavelength, γ-radiation has the highest penetration power. Sterilization by γ-irradiation of disposable plastic ware enables a high degree of sterility. This facilitates the maintenance of sterility for prolonged periods until packed properly.
Financial aspects and environmental impacts
Over the past decade, one of the key issues raised universally has been that of sustainable development; ensuring that future generations will enjoy the same standard of living and opportunities to development that we currently enjoy. Both public and private organizations are increasingly under pressure to evaluate their current processes in order to pinpoint and reduce potential sources of waste throughout their supply chains. Identifying whether landfilling, incineration, or reuse/recycling is preferable comprises various methodological issues. This will relate to the assurance of both social and financial effects identified with the different alternatives in a Life Cycle Assessment (LCA) and the monetary valuation of these effects in a cost-benefit analysis. This evaluation must be done keeping in mind to recover as much energy as possible with every other alternative involved. In the cost-benefit analysis, the environmental impacts related to various aspects of waste management such as collection, sorting, transport, reuse, recycling, landfilling, and incineration were translated into monetary values. This allows the aggregation of and the comparison between financial and environmental impacts and cost-benefits of the various options considered. All of these techniques imply some degree of uncertainty as it is usually difficult to establish exact impacts for various aspects. The literature on LCA of plastic waste management systems is immense and the results reported are generally consistent, showing that reuse/recycling greatly reduces the environmental impact on global warming potential and total energy use.
Between 1950 and 2015, the total amount of waste accumulated due to both primary and secondary (recycled) plastic sources was equated to 6300 Mt [25]. Of this, around 800 Mt (12%) of plastics have been incinerated and 600 Mt (9%) have been recycled, only 10% of which have been recycled more than once. Around 4900 Mt (60% of all plastics ever produced) were disposed of and have been accumulating in landfills or in the environment. Globally, Europe and China have been found to have the highest plastic recycling rates with 30 and 25% respectively in 2014, whereas the plastic recycling rate of the United States remained steady at 9% since 2012. However, reuse/recycling delays, rather than avoid, final disposal. Decreasing current global incineration and recycling rates by 5%, and adjusting the time trends accordingly, decreases the cumulative discarded plastic waste by 6%. But, the benefits of recycling can often be offset by the excessive energy consumption associated with waste management.
A detailed study of alternatives to drinking water supply has shown that washing and reusing containers has far lower global warming potential impacts than recycling single-use PET bottles [26]. This report has found that reusing released 79% percent less greenhouse gas emissions whereas recycling released only 16% less compared with waste disposal. It implies that reuse is fivefold more efficient in reducing greenhouse gas emissions and it is in the worst-case scenario. Also, they compared the “best-case scenario” of reuse and recycling and found that reuse had 98% less global warming potential impact than that of recycling. Reuse is far superior to recycling.
One of the sustainable goals is to recycle the material over as many times as possible before the recycling becomes a technically energetically unfavorable option. Unfortunately, in the case of PS recycling is not easily affordable economically and often leads to end up in landfills. Alternatively, it can be reused multiple times before disposing of. Since PS is made from fossil fuel feedstock, it has a relatively high net calorific value i.e. the potential energy inherent in the waste feedstock which can be utilized by combustion. The high amount of energy generated by combustion could be recovered by using it as a fuel. This approach demonstrates a cradle-to-cradle approach as it recovers as much energy as possible and nothing goes to the landfills.
The main limitations of this technique are that the RTPS becomes fragile after multiple irradiations and that color changes have been observed limiting the reuse of material to no more than three times before discarding. Materials capable of withstand multiple irradiation steps can be optimally used for reuse before ending up in landfills. In addition, the relatively extra work involved in the process, such as washing, maintaining clean plates, and re-irradiation makes scientists reluctant towards it. Most laboratories do not have irradiation facilities which makes this process relatively laborious and cost ineffective. Once the strategy is established, it becomes possible to reuse not only petri dishes but also other disposable products used regularly in laboratories. It is therefore necessary to develop tools for the rational evaluation and comparison of alternatives for the collection, separation, and treatment of waste fractions. This reusing process provides a feasible solution both environmentally and economically compared to disposing of PS including incineration and landfill dumping with reduced damaging environmental impacts.
Conclusion
This study provides evidence for the reuse of disposable polystyrene (PS) Petri dishes by sterilization using γ-irradiation. The UV-Vis spectrometry and pH analyses clearly suggest that by re-irradiation there are no significant structural and chemical modifications at higher doses (51, 77, and 129 kGy) enabling the reuse. Thus, reuse not only reduces waste generation but also reduces experimental costs. This technique is effective over recycling and disposal of PS including landfilling and incineration with reduced environmental impacts. It is possible to reuse not only petri dishes but also other disposable products used regularly in laboratories. We suggest that end-of-life PS products could be used as a fuel resource leading to the recovery of much energy by combustion because of its high calorific value. Thus, provides a cradle-to-cradle approach, where there would not be any waste generation and nothing goes to the landfills offering a sustainable alternative to recycling and disposal of PS.
We gratefully acknowledge Mr. P. Thillaimani (Technical Officer), CIF, Pondicherry University for helping in gamma irradiation.
- Plastics Europe. Plastics - the Facts 2014/15: An analysis of European latest plastics production, demand and waste data. Brussels, Belgium: EuPC; 2015.
- Urbina MA, Watts AJ, Reardon EE. Environment: Labs should cut plastic waste too. Nature. 2015 Dec 24;528(7583):479. doi: 10.1038/528479c. PMID: 26701046.
- Sheehan B. Greenhouse Gas Impacts of Disposable vs Reusable Foodservice Products. Technical Report; 2017.
- Grassië N, Weir N. The photooxidation of polymers. II. Photolysis of polystyrene. J Appl Polym Sci. 1965;9(3):975–986.
- Yeon YH, Shim HE, Park JH, Lee NH, Park JY, Chae MS, Mun JH, Lee JH, Gwon HJ. Evaluation of Radiation Resistance of Polystyrene Using Molecular Dynamics Simulation. Materials (Basel). 2022 Jan 4;15(1):346. doi: 10.3390/ma15010346. PMID: 35009493; PMCID: PMC8746079.
- Geuskens G, Baeyens-Volant D, Delaunois G, Lu-Vinh Q, Piret W, David C. Photo-oxidation of polymers—I. Eur Polym J. 1978;14(4):291–297.
- Kassem M, Bassiouni M, El-Muraikhi M. The effect of γ-irradiation on the optical properties of polyoxymethylene compacts. J Mater Sci Mater Electron. 2002; 13:717–719.
- Parashar P, Datt S. Effect of gamma-ray irradiation on the mechanical strength of polystyrene-ferrocene composite. Polym Test. 1993; 12(5):429–435.
- Tian Q, Takács E, Krakovský I, Horváth ZE, Rosta L, Almásy L. Study on the microstructure of polyester polyurethane irradiated in air and water. Polymers. 2015; 7(9):1755–1766.
- Williams JL, Dunn TS. Advances in the radiation stabilization of polypropylene. Radiat Phys Chem. 1983; 22(1–2):209–214.
- Vardhan PV, Shukla LI. FT-IR investigations on effect of high doses of gamma radiation-induced damage to polystyrene and mechanism of formation of radiolysis products. Radiat Environ Biophys. 2018 Aug;57(3):301-310. doi: 10.1007/s00411-018-0740-y. Epub 2018 Apr 17. PMID: 29666924.
- Buchalla R, Boess C, Bögl KW. Analysis of volatile radiolysis products in gamma-irradiated LDPE and polypropylene films by thermal desorption-gas chromatography-mass spectrometry. Appl Radiat Isot. 2000 Feb;52(2):251-69. doi: 10.1016/s0969-8043(99)00125-6. PMID: 10697736.
- Ward BD. Absorption spectra of aromatic free radicals: a vibrational analysis of the 3050 Å absorption spectrum of benzyl and a new transition of phenoxyl. Spectrochim Acta. 1968; 24(7):813–818.
- McGovern J, Grim JM, Teach WC. Determination of monomer in polystyrene. Anal Chem. 1948; 20(4):312–314.
- Newell JE. Residual monomer in polystyrene. Anal Chem. 1951; 23(3):445–447.
- Li T, Zhou C, Jiang M. UV absorption spectra of polystyrene. Polym Bull. 1991; 25(2):211–216.
- Rabaeh KA, Basfar AA. A polystyrene film dosimeter containing dithizone dye for high dose applications of gamma-ray source. Radiat Phys Chem. 2020; 170:108646.
- Abdel-Hady EE, Abdel-Hamid HM, Mohamed HFM. Electron beam and gamma irradiation effects on conducting polystyrene studied by positron annihilation technique. Radiat Meas. 2004; 38(2):211–216.
- Lee JY, Kim YN, Kim BH, Kim SO, Cho SO. Fabrication of luminescent nanoarchitectures by electron irradiation of polystyrene. Adv Mater. 2008; 20(11):2094–2098.
- Whitaker H, Johnson KF. Pre-irradiation environmental effects on radiation damage of polystyrene. Radiat Phys Chem. 1993; 41(1–2):121–126.
- De Castro Monsores KG, Da Silva AO, De Sant’ Ana Oliveira S, Weber RP, Filho PFS, Monteiro SN. Influence of ultraviolet radiation on polystyrene. J Mater Res Technol. 2021; 13:359–365.
- Zagorski ZP, Głuszewski W. Irreversible radiolytic dehydrogenation of polymers: the key to recognition of mechanisms. INCT Annu Rep. 2004:40–42.
- Chang Z, LaVerne JA. Hydrogen production in γ-ray and helium-ion radiolysis of polyethylene, polypropylene, poly(methyl-methacrylate), and polystyrene. J Polym Sci A. 2000; 38(9):1656–1661.
- Abu Saleh BA, Elimat ZM, Alzubi RI, Juwhari HK, Zihlif AM. Ultrafine iron particles/polystyrene composites: Effects of gamma radiation and manufacture aging on the AC electrical characterization. Radiat Eff Defects Solids. 2022; 177(9-10):1065-1074.
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017 Jul 19;3(7):e1700782. doi: 10.1126/sciadv.1700782. PMID: 28776036; PMCID: PMC5517107.
- Allaway D. Comparing Prevention, Recycling, and Disposal: A supplement to DEQ’s Life Cycle Assessment of Drinking Water Delivery Systems: Bottled Water, Tap Water, and Home/Office Delivery Water. Oregon Department of Environmental Quality; 2009.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
