Archives of Sports Medicine and Physiotherapy
Development of Glucocorticoid-Induced and Exercise-Caused Myopathies
Teet Seene*, Karin Alev and Priit Kaasik
Cite this as
Seene T, Alev K, Kaasik P (2019) Development of Glucocorticoid-Induced and Exercise-Caused Myopathies. Arch Sports Med Physiother 4(1): 005-009. DOI: 10.17352/asmp.000010The aim of this short review is to analyze the pathogenic factors induce glucocorticoid and exercise myopathies and to show whether exercise myopathy is the mild form of glucocorticoid myopathy as was hypothesized by prof. Lehmann about two decades ago.
These pathogenic factors are high level of blood corticosteroids, structural changes in skeletal muscle fibers, decreased protein synthesis and increased degradation rate, slow turnover rate of contractile proteins and slow regeneration of muscle tissue due to the decrease in the number of satellite cells under the basal lamina of muscle fibers. Despite of apparent similarity in the destruction process of the myofibrillar apparatus, the development of these myopathies are different. Argument about the high corticosteroid level in case of exercise myopathy is not absolutely correct as during exhaustive exercise the level of this hormone in blood is high only for a short time. In case of exercise myopathy the destruction of myofibrils mainly occurs in muscle fibres with higher oxidative capacity (FT O-G and ST O), in case of glucocorticoid myopathy the respective fibre type is FT G. Disarray of myosin filaments begins in case of both glucocorticoid and exercise myopathy from the peripheral myofibrils, however, as mentioned earlier, in different fibre types. The similar features in both myopathies include the decrease of contractile proteins synthesis rate, increase of degradation rate, slow turnover rate and regeneration. Analysis of the relevant pathogenic factors confirms that the above described pathogenesis in glucocorticoid and exercise myopathies occur in different fibre types. In conclusion, there is sufficient ground to conclude that exercise-induced myopathy in type O-G and O muscle fibres occurs with less destruction in comparison with glucocorticoid-induced myopathy in type G fibres, however, this does not prove exercise myopathy to be the mild form of glucocorticoid myopathy.
Introduction
About two decades ago Prof. Manfred Lehmann [1] hypothesized that exhaustive chronic exercise is the mild form of glucocorticoid-induced myopathy. This hypothesis is based on the common findings in Cushing’s syndrome, and high blood corticosteroid level established in glucocorticoid-supplemented and overstrained laboratory animals, and overstrained athletes [2]. There at the elevated hormone level proposed to be the main reason of development of the described myopathies [1]. In addition, depressed contractile proteins turnover, increased catabolic and decreased anabolic processes in skeletal muscle, particularly in the myofibrillar compartment are important factors in the development of the above mentioned myopathies [3-7]. There are many similarities in the mechanisms of glucocorticoid-induced and exercise-induced myopathies, but also a great number of differences (Fig. 1 and 2). Analysis of the pathogenic factors on what the hypothesis is based on, should show whether the features that characterize both forms of myopathies really exist or only seemingly to be similar.
The aim of this short review is to analyze the pathogenic factors induce glucocorticoid and exercise myopathies and to show whether exercise myopathy is the mild form of glucocorticoid myopathy as was hypothesized by prof. Lehmann about two decades ago.
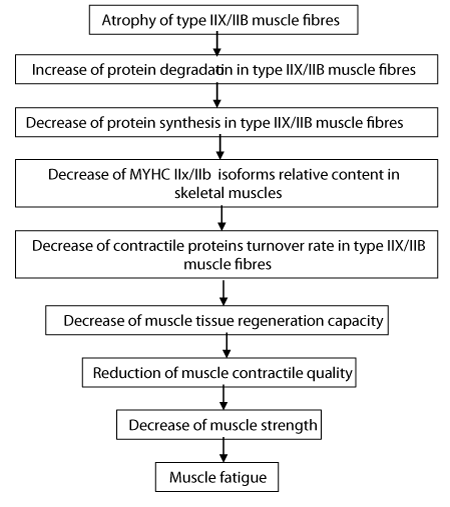
Glucocortcoid-Induced Myopathy
Fast twitch (FT) muscle fibres and their myofibrils are thinner in glucocorticoid-induced myopathic muscle compared to the sedentary group, thin and thick filaments have disappeared completely from one fifth of the area of myofibrils [7,8]. The intensive destruction of myofibrils and degradation of contractile proteins, particularly myosin heavy chain (MyHC) IIb isoform [9,10], are the main reasons for reduced muscle strength, motor activity, and weakness in glucocorticoid-induced myopathic rats [10,11]. The destruction of myofibrils begins in the myosin filaments of peripheral glycolytic muscle fibres, and then spreads all over the myofibrillar apparatus [12,13]. Another reason is the slower myofibrillar protein synthesis rate and assembly of thick and thin filaments. The decrease in the relative content of the MyHC IIb isoform and the respective increase of the MyHC IId isoform show that quantitative changes in myofibrils are significantly related to the qualitative remodeling of thick myofilaments in myopathic glycolytic muscle fibres [8,10]. Changes in the myofibrils ultrastructure of myopathic muscle fibres are also related to the functional modification of glycolytic muscle fibres. These modifications have not been observed in slow twitch (ST) oxidative muscle fibres [10]. Glucocorticoid-caused wasting is a result of the loss of FT fibres, their myofibrils, contractile proteins, and does not depend on the age [11]. The excess of glucocorticoids decreases the skeletal muscle regeneration and correlates with a decrease in satellite cells number under the basal lamina of skeletal muscle fibres [5,13-15]. The intensity of the regeneration of skeletal muscle depends on the mass of muscle and its contractile properties [16]. Glucocorticoid myopathy induces structural changes in the ultrastructure of satellite cells [12,17], these changes are similar to those occurring in skeletal muscle fibres where the satellite cells are located [4, 13, 14]. Decrease in the number of satellite cells and changes in their ultrastructure cause decreased regeneration capacity in glucocorticoid-induced myopathic muscle [8].There is positive correlation between muscle atrophy and elasticity, and negative correlation between the state of atrophy and muscle tone [18]. Decrease in contractile protein myosin and in elastic proteins titin and nebulin leads to the reduction of muscle elasticity and the generation of tension in myopathic muscle [18]. Protein degradation in skeletal muscle fibres, particularly in FT fibres with low oxidative capacity, is mediated by the activity of ubiquitin–proteosomal and lysosomal pathways [19]. The activity of ubiquitin–proteosomal pathway is significantly increased in atrophying muscle due to transcriptional activation of E3 ligase-encoding genes atrogin-1 and MuRF 1 [20]. A glucocorticoid receptor in skeletal muscle (REDD1, KLF15) inhibits mTOR activity via BCAT 2 gene activation. KLF15 upregulates the expression of E3 ubiquitin ligases atrogin-1 and MuRF 1, causing atrophy in the muscle fibre [20]. The ubiquitin–proteasome pathway, satellite cells in muscle, the function of related receptors and signalling pathways influence this process by tumor-induced systematic inflammation [21].
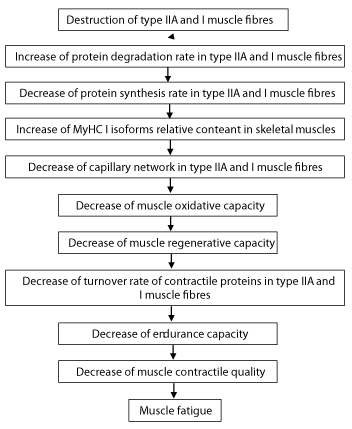
Exercise-induced myopathy
As a result of exhaustive exercise (stress > recovery imbalance) develops the overtraining syndrome with symptoms of myopathy [1]. Exercise-induced myopathy is accompanied by the decreased synthesis rate of muscle proteins, particularly myofibrillar proteins, and the increased protein degradation rate in skeletal muscle [13,22,23,14].The process of destruction in myofibrils occurs in volume-induced overtrained skeletal muscles, mainly in FT oxidative glycolytic (O-G) muscle fibres and in ST oxidative (O) muscle fibres [13,14,17]. The relative content of MyHC I isoform in ST muscle fibres increases and IIa isoform decreases in exercise-caused myopathic muscles. In FT muscle fibres the relative content of MyHC IIb isoform decreases and IIa isoform increases [14,17,22,23,17]. These changes in MyHC isoforms show that contractile properties of ST and FT muscles change in different ways in accordance with the oxidative capacity of muscle [8,24]. In myopathic muscle the changes in myosin light chain (MyLC) isoforms are considerably smaller in comparison with subsequent changes in MyHC isoforms [4,17,22,23]. The most significant changes in MyLC isoforms appear in FT muscle fibres. The regeneration of MyHC IIb and MyLC 1f isoforms, having high affinity to each other in FT muscle fibres after tissue damage, proceeds at different speed [25]. MyLC 3f isoform regenerates faster than MyHC IIb isoform in FT muscle fibres with low oxidative capacity. It has been shown that MyLC 1 isoform can negatively affect myoblast proliferation [26]. In exercise-caused myopathic muscles myofibril cross sectional area (CSA) in type FT O-G fibres decreased 33% and in type FT G fibres 44% [17]. Protein degradation rate increased in both type O-G and G fibres, 63% and 69% respectively, in comparison with the control group [17]. Myofibrils in both types of FT myopathic muscle fibres are significantly thinner as the result of more intensive protein degradation. Regeneration capacity is higher in type FT O-G fibres than in type Ft G fibres due to the presence of satellite cells [13,17]. Structural changes in exercise-caused myopathic muscle are associated with calcium overload, free radical formation, the decrease in energy supply and the reduction in the muscle defense system [27,28]. Exhaustive exercise is associated with enhanced oxygen consumption in skeletal muscles, increased lipid peroxidation and inhibition of key mitochondrial enzymes [29,30], as well as immune reaction, metabolic and cellular signal transduction and increasing rate of heat shock proteins (HSP) synthesis [31-33]. The decrease in insulin-like growth factor-1 (IGF-1) and mechano growth factor (MGF) results in slow regeneration of exercise-induced myopathic muscles [34-36]. Increased muscle protein degradation and decreased synthesis rate in myopathic skeletal muscle, as well as changes in MyHC isoform pattern are fibre-type specific [22]. Regulatory protein Tn-T and minor C-protein are sensitive to the increase in training volume and together with MyHC isoforms play the key role in the changes of functional properties of contractile machinery in exercise-induced myopathic skeletal muscle [22,23,37].
Comparison of changes in glucocorticoid- and exercise-induced myopathic muscles
Decrease in CSA of muscle fibres and myofibrils has been observed in case of both glucocorticoid-caused and exercise-caused myopathies [1,7,17]. Decrease in protein synthesis, increase in protein degradation rate and slow protein turnover rate are also in principle comparable in both types of myopathies [5,10,14,38]. As mentioned in the Introduction, the Lehmann’s hypothesis is based on the similarities in the level of corticosteroids in blood and on the structural changes in skeletal muscle occurring during glucocorticoid- and exercise-induced myopathies [1]. Despite similarities in the process of destruction of the myofibrillar apparatus, the respective myopathies develop in different muscle fibre types [10,22,14]. Therefore, the main argument of the hypothesis, high level of corticosteroids during exhaustive exercise, is not conclusive, since this hormone level is maintained during a relatively short period and decreases in the recovery period [3]. The destruction of myofibrils has been registered in glucocorticoid-caused myopathic FT G muscle fibres [13,39] and in exercise- caused myopathic FT O-G fibres [6,39,13]. This difference between the two types of myopathies at muscle fibre level is the real one. Imaginary similarity in the process of destruction of myofibrils in both types of myopathies is the disarray of myosin filaments from the periphery of myofibrils [13] since it occurs in different fibre types and their neuromuscular junctions [13,14]. Muscle fibres with higher oxidative capacity are more susceptible to oxidative damage by reactive oxygen species, compared to fibres with low oxidative capacity and predominantly with MyHC IIb and IId isoforms. Higher oxidative capacity of muscle fibres makes them more resistant to the degradation of muscle proteins, including in the myopathic muscle. During overtraining, type IIA muscle fibres are recruited more frequently, and there are also notable structural destructions [28]. Due to the relatively high regenerative capacity of type IIA fibres, they can maintain low-intensity muscle contraction. Type I and IIA muscle fibres that have higher oxidative capacity are relatively resistant to the degradation of myofibrillar proteins [24,40].
In conclusion, high level of corticosteroids in blood, decrease of contractile proteins synthesis rate, increase of degradation rate, slow turnover rate and regeneration are similar in case of both myopathies. The analyzis of relevant pathogenic factors proves that the above processes occur in different fibre types (Figures 1,2) and there is sufficient ground to conclude that exercise-induced myopathy in type ST O and FT O-G fibres occurs more mildly (fibres are with higher oxidative capacity) in comparision with glucocorticoid-induced myopathy in type FT G fibres (fibres are with low oxidative capacity), but this fact does not prove that exercise-caused myopathy is the mild form of glucocorticoid-caused myopathy.
Conclusion
The destruction of myofibrils has been registered in glucocorticoid-caused myopathic FT G muscle fibres and in exercise- caused myopathic FT O-G fibres. This is real difference between the two types of myopathies at muscle fibre level. Imaginary similarity appeare in the process of destruction of myofibrils in both types of myopathies: disarray of myosin filaments from the periphery of myofibrils since it occurs in different fibre types. and their neuromuscular junctions [33,34]. Muscle fibres with higher oxidative capacity are more susceptible to oxidative damage by reactive oxygen species, compared to fibres with low oxidative capacity and predominantly with MyHC IIb and IId isoforms. Higher oxidative capacity of muscle fibres makes them more resistant to the degradation of muscle proteins, including in the myopathic muscle. During overtraining, type IIA muscle fibres are recruited more frequently, and there are also notable structural destructions [31]. Due to the relatively high regenerative capacity of type FT O-G fibres, they can maintain low-intensity muscle contraction. ST O and FT O-G muscle fibres that have higher oxidative capacity are relatively resistant to the degradation of myofibrillar proteins [24,30]. So, high level of corticosteroids in blood, decrease of contractile proteins synthesis rate, increase of degradation rate, slow turnover rate and regeneration are similar in case of both myopathies. The analyzis of relevant pathogenic factors proves that the above processes occur in different fibre types and there is sufficient ground to conclude that exercise-induced myopathy in ST O and FT O-G fibres occurs more mildly (fibres with higher oxidative capacity) in comparision with glucocorticoid-induced myopathy in FT G fibres (fibres with low oxidative capacity), but this fact does not prove that exercise-caused myopathy is the mild form of glucocorticoid-caused myopathy.
This study was supported by the Estonian Research Council, Research project number TKKSB 1787 ??? IUT20-58 ??? TMVSF14058I ?????.
We would like to thank Mrs Piret Pärsim for technical xpertise.
- Lehmann M, Baur S, Gastmann U, Liu Y, Lormes W, et al. (1999) Selected parameters and mechanisms of peripheral and central fatigue and regeneration in overtrained athletes. In: Lehmann M, et al. (eds). Overload, Performance Incompetence, and Regeneration in Sport, Kluwer Academic/ Plenum Press, New York. 7–25.
- Lehmann M, Baur S, Netzer N, Gastmann U (1997) Monitoring high-intensity endurance training using neuromuscular excitability to recognize overtraining. Eur J Appl Physiology 76: 187-191. Link: https://bit.ly/2Y94tT4
- Seene T, Viru A (1982) The catabolic effect of glucocorticoids on different types of skeletal muscle fibres and its dependence upon muscle activity and interaction with anabolic steroids. J Steroid Biochem 16: 349–352. Link: https://bit.ly/2Sv6Nm2
- Seene T, Kaasik P, Umnova M (2009) Structural rearrangements in contractile apparatus and resulting skeletal muscle remodelling: effect of exercise training. J Sports Med Phys Fitness 49: 410−423. Link: https://bit.ly/2Y2t8sw
- Seene T (1994) Turnover of skeletal muscle contractile proteins in glucocorticoid myopathy. J Steroid Biochem Mol Biol 50: 1–4. Link: https://bit.ly/2Ss9a9t
- Kaasik P, Seene T (2010) The overtraining syndrome: reflexion in skeletal muscle. Gazzetta Medica Italiana Archivio Per Le Scienze Mediche 169: 311-319. Link: https://bit.ly/2Ojraoa
- Kaasik P, Umnova M, Alev K, Selart A, Seene T (2012) Fine architectonics and protein turnover rate in myofibrils of glucocorticoid caused myopathic rats. J Interdiscip Histopathol 1: 5–10. Link: https://bit.ly/2JKvs3x
- Seene T, Kaasik P (2016) Role of myofibrillar protein catabolism in development of glucocorticoid myopathy: Aging and functional activity aspects. Metabolites 6: 15. Link: https://bit.ly/2Y5QZrh
- Fernandez-Rodriques E, Stewart P, Cooper M (2009) The pituitary-adrenal axis and body composition. Pituitary 12: 105–115. Link: https://bit.ly/2M9FKff
- Seene T, Kaasik P, Pehme A , Alev K, Riso EM (2003) The effect of glucocorticoids on the myosin heavy chain isoforms’ turnover in skeletal muscle. J Steroid Biochem Mol Biol 86: 201–206. Link: https://bit.ly/2Z2wr4l
- Kaasik P, Umnova M, Pehme A, Alev K, Aru M, et al. (2007) Ageing and dexamethasone associated sarcopenia: Peculiarities of regeneration. J. Steroid Biochem Mol Biol 105: 85–90. Link: https://bit.ly/2JKDh9i
- Seene T, Umnova M, Alev K, Pehme A (1988) Effect of glucocorticoids on contractile apparatus of rat skeletal muscle. J Steroid Biochem 29: 313–317. Link: https://bit.ly/30OxcOU
- Seene T, Umnova M, Kaasik P (1999) The exercise myopathy. In: Lehmann M et al (eds) Overload, Performance Incompentence and Regeneration in Sport. Kluwer Academic/Plenum Publishers119–130.
- Seene T, Umnova M, Kaasik P, Alev K, Pehme A (2008) Ovetraining injuries in athletic population. In: Tiidus PM (ed). Skeletal muscle damage and repair. Champaign (IL): Human Kinetics 173–184.
- Shultz E, Darr K (1990) The role of satellite cells in adaptive or induced fiber transformations. In: The Dynamics State of Muscle Fibers; Pette D. (ed) Walter de Gruyter: Berlin, Germany 667–681. Link: https://bit.ly/30Pom3j
- Järva J, Alev K, Seene T (1997) Myosin heavy chain composition in regulating skeletal muscle grafts. Basic Appl Myol 7: 137–141. Link: https://bit.ly/2SvvD5n
- Kaasik P, Umnova M, Seene T (2014) Exercise myopathy: Changes in myofibrils of fast-twitch muscle fibres.Biol.Sport 31: 167-171. Link: https://bit.ly/2JJA8qe
- Aru M, Alev K, Gapeyeva H, Vain A, Puhke R, et al. (2013) Glucocorticoid-induced alterations in titin, nebulin, myosin heavy chain isoform content and viscosoelastic properties of rat skeletal muscle. Adv Biol Chem 3: 70–75. Link: https://bit.ly/2MbiAoq
- Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23: 160–170. Link: https://bit.ly/2y4qEPC
- Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, et al. (2011) Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab 13: 170–182. Link: https://bit.ly/2SvJXL7
- Fearon K, Evans WJ, Anker SD (2011) Myopenia—A new unviversal term for muscle wasting. J. Cachexia Sarcopenia Muscle 2: 1–3. Link: https://bit.ly/30RP8b9
- Seene T, Alev K, Kaasik P, Pehme A, Parring AM (2005) Endurance training: volume-dependent adaptational changes in myosin. Int J Sports Med 26: 815–821. Link: https://bit.ly/2OaG9Ar
- Seene T, Kaasik P, Alev K, Pehme A, Riso EM (2004) Composition and turnover of contractile proteins in volume-overtrained skeletal muscle. Int J Sports Med 25: 438–445. Link: https://bit.ly/30PprrT
- Seene T, Kaasik P, Seppet E (2017) Changes in myofibrillar and mitochondrial compartments during increased activity: Dependance from oxidative capacity of muscle. Health 9: 779- 798. Link: https://bit.ly/2y4s9NK
- Alev K, Kaasik P, Pehme A, Aru M, Parring AM, et al. (2009) Physiological role of myosin light and heavy chain isoforms in fast- twitch and slow-twitch muscles: Effect of exercise. Biol Sport 26: 215-234. Link: https://bit.ly/2M3MScJ
- Zhang SZ, Xu Y Xie HQ, Li XQ, Wei YQ, Yang ZM (2009) The possible role of myosin light chain in myoblast proliferation. Biol Res 42: 121-132. Link: https://bit.ly/2ZeGJy4
- Lowery L, Forsythe C (2006) Protein and overtraining: Potential application for free-living athletes. J Int Soc Sports Nutr 3: 42-50. Link: https://bit.ly/2Y6ielv
- Seene T, Kaasik P, Seppet E (2017) Crosstalk between mitochondria and myofibrils in adult and aging striated muscle tissue: Effect of increased functional activity. Asian J Res Med Pharm Sci 1: 1-13. Link: https://bit.ly/2M1k7xk
- Halliwell B, Gutteridge JMC (2007) Free Radical Biology and Medicine. Oxford: Clarendon Press. Link: https://bit.ly/2K1ekFI
- UIso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189: 41-54. Link: https://bit.ly/2xZkUH7
- Pansarasa O, D'Antona G, Gualea MR, Marzani B, Pellegrino MA, et al. (2002) Oxidative stress: effects of mild endurance training and testosterone treatment on rat gastrocnemius muscle. Eur J Appl Physiol 47: 550- 555. Link: https://bit.ly/2OjxRXk
- Fehrenbach E, Niess AM (1999) Role of heat shock proteins in the exercise response. Exerc Immunol Rev 5: 57-77. Link: https://bit.ly/2XRva3k
- Moseley PL (2000) Exercise, stress, and the immune conversion. Exerc Sport Sci Rev 28: 128-132. Link: https://bit.ly/30RsOib
- Goldspink G (2000) Cloning of local growth factors involved in the determination of muscle mass. Br J Sports Med 34: 159-160. Link: https://bit.ly/2y0QgNt
- Goldspink G (1999) Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle ln response to stretch and overload. J Anat 194: 323-334. Link: https://bit.ly/2YckU5e
- Monnier JF, Benhaddad AA, Micallef JP, Mercier J, Brun JF (2000) Relationships between blood viscosity and insulin-like growth factor I status in athletes. Clin Hemorhreol Microcirc 22: 277-286. Link: https://bit.ly/30QlqDD
- Morton JP, Kayani AC, McArdle A, Drust B (2009) The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39: 643-662. Link: https://bit.ly/2y23uK0
- Seene T, Kaasik P, Alev K (2011) Muscle protein turnover in endurance training: a review. Int J Sports Med 32: 905−911. Link: https://bit.ly/2JIXtIv
- Seene T, Kaasik P, Riso EM (2012) Review on aging, unloading and reloading: changes in skeletal muscle quantity and quality. Arch Gerontol Geriatr 54: 374-380. Link: https://bit.ly/2GlhP8U
- Seene T, Kaasik P (2013) Muscle damage and regeneration: Response to exercise training. Health 5: 136-145. Link: https://bit.ly/2O9MxrE
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
